[English] 日本語
 Yorodumi
Yorodumi- EMDB-33370: Cryo-EM structure of the T=3 lake sinai virus 2 virus-like capsid... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the T=3 lake sinai virus 2 virus-like capsid at pH 6.5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | VIRUS LIKE PARTICLE | |||||||||
| Function / homology | virion component / Viral coat protein subunit / Capsid protein alpha Function and homology information Function and homology information | |||||||||
| Biological species |  Lake Sinai virus 2 Lake Sinai virus 2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.74 Å | |||||||||
 Authors Authors | Chen NC / Wang CH / Chen CJ / Yoshimura M / Guan HH / Chuankhayan P / Lin CC | |||||||||
| Funding support |  Taiwan, 1 items Taiwan, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structures of honeybee-infecting Lake Sinai virus reveal domain functions and capsid assembly with dynamic motions. Authors: Nai-Chi Chen / Chun-Hsiung Wang / Masato Yoshimura / Yi-Qi Yeh / Hong-Hsiang Guan / Phimonphan Chuankhayan / Chien-Chih Lin / Pei-Ju Lin / Yen-Chieh Huang / Soichi Wakatsuki / Meng-Chiao Ho / Chun-Jung Chen /   Abstract: Understanding the structural diversity of honeybee-infecting viruses is critical to maintain pollinator health and manage the spread of diseases in ecology and agriculture. We determine cryo-EM ...Understanding the structural diversity of honeybee-infecting viruses is critical to maintain pollinator health and manage the spread of diseases in ecology and agriculture. We determine cryo-EM structures of T = 4 and T = 3 capsids of virus-like particles (VLPs) of Lake Sinai virus (LSV) 2 and delta-N48 LSV1, belonging to tetraviruses, at resolutions of 2.3-2.6 Å in various pH environments. Structural analysis shows that the LSV2 capsid protein (CP) structural features, particularly the protruding domain and C-arm, differ from those of other tetraviruses. The anchor loop on the central β-barrel domain interacts with the neighboring subunit to stabilize homo-trimeric capsomeres during assembly. Delta-N48 LSV1 CP interacts with ssRNA via the rigid helix α1', α1'-α1 loop, β-barrel domain, and C-arm. Cryo-EM reconstructions, combined with X-ray crystallographic and small-angle scattering analyses, indicate that pH affects capsid conformations by regulating reversible dynamic particle motions and sizes of LSV2 VLPs. C-arms exist in all LSV2 and delta-N48 LSV1 VLPs across varied pH conditions, indicating that autoproteolysis cleavage is not required for LSV maturation. The observed linear domino-scaffold structures of various lengths, made up of trapezoid-shape capsomeres, provide a basis for icosahedral T = 4 and T = 3 architecture assemblies. These findings advance understanding of honeybee-infecting viruses that can cause Colony Collapse Disorder. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33370.map.gz emd_33370.map.gz | 482.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33370-v30.xml emd-33370-v30.xml emd-33370.xml emd-33370.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33370.png emd_33370.png | 98.9 KB | ||
| Filedesc metadata |  emd-33370.cif.gz emd-33370.cif.gz | 5.5 KB | ||
| Others |  emd_33370_half_map_1.map.gz emd_33370_half_map_1.map.gz emd_33370_half_map_2.map.gz emd_33370_half_map_2.map.gz | 473.6 MB 482.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33370 http://ftp.pdbj.org/pub/emdb/structures/EMD-33370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33370 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33370 | HTTPS FTP |
-Validation report
| Summary document |  emd_33370_validation.pdf.gz emd_33370_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33370_full_validation.pdf.gz emd_33370_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_33370_validation.xml.gz emd_33370_validation.xml.gz | 19 KB | Display | |
| Data in CIF |  emd_33370_validation.cif.gz emd_33370_validation.cif.gz | 22.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33370 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33370 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33370 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33370 | HTTPS FTP |
-Related structure data
| Related structure data |  7xpdMC  7xpaC  7xpbC  7xpeC  7xpfC  7xpgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33370.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33370.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0537 Å | ||||||||||||||||||||||||||||||||||||
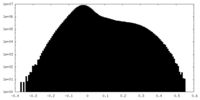
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33370_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
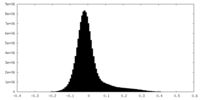
| Density Histograms |
-Half map: #2
| File | emd_33370_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Lake Sinai virus 2
| Entire | Name:  Lake Sinai virus 2 Lake Sinai virus 2 |
|---|---|
| Components |
|
-Supramolecule #1: Lake Sinai virus 2
| Supramolecule | Name: Lake Sinai virus 2 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 1041831 / Sci species name: Lake Sinai virus 2 / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: Yes |
|---|
-Macromolecule #1: Capsid protein alpha
| Macromolecule | Name: Capsid protein alpha / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Lake Sinai virus 2 Lake Sinai virus 2 |
| Molecular weight | Theoretical: 57.344309 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNPPTTTTTT TRTIRAPKVQ LTPNSATRRR RNRRRRRPAA AIAGPLSIQP SSINRRMVSR VTRRSAVVTA AGLAWLRQYL NPMGPDTTS VTGYPDGSAV TTCIADYSNT FNVSFPPREA LYCTGSSSSE KPTLVDADNY AKIDKWSNYD ITLCVLALPM L RNVVMLRL ...String: MNPPTTTTTT TRTIRAPKVQ LTPNSATRRR RNRRRRRPAA AIAGPLSIQP SSINRRMVSR VTRRSAVVTA AGLAWLRQYL NPMGPDTTS VTGYPDGSAV TTCIADYSNT FNVSFPPREA LYCTGSSSSE KPTLVDADNY AKIDKWSNYD ITLCVLALPM L RNVVMLRL YPHTPTAFAL TEQTPNFPQR FPNWSVYSAD GTRFNNGDEP GYLQSYVYLP NVDKHLSAAR GYRLLSRGIT GI FSAPALE TQGFVTACQY LAEGSIQSQS IKSDAVRSVT VNSDGTVKNV ESSSQTVSSM PRYVFPLDGD NCAPSSLTET YHQ AYQSKA TDGFYMPVLS SSRDNPFHPP QPRAIAVYGS FLARGCLDPV SEAHEADGPT HDIYRLNVAD DVAPLFNTGV VWFE GISPK FSLKLKTRTV LQYIPTSGSV LANFTRHEPT YDQIALDAAD RLRNLMPHAY PAAYNDWGWL GDLLDSAISM LPGVG TVYN IAKPLIKPAW NWLGNKVSDF FGNPVARDGD IFFDAK UniProtKB: Capsid protein alpha |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.72 µm / Nominal defocus min: 0.25 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


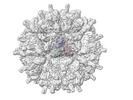
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)