+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of EDS1 and SAG101 with ATP-APDR | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NLR / Plant Protein / Plant immune signaling | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of defense response to oomycetes / positive regulation of leaf senescence / aerenchyma formation / EDS1 disease-resistance complex / leaf abscission / systemic acquired resistance / systemic acquired resistance, salicylic acid mediated signaling pathway / positive regulation of defense response to bacterium / response to singlet oxygen / plant-type hypersensitive response ...positive regulation of defense response to oomycetes / positive regulation of leaf senescence / aerenchyma formation / EDS1 disease-resistance complex / leaf abscission / systemic acquired resistance / systemic acquired resistance, salicylic acid mediated signaling pathway / positive regulation of defense response to bacterium / response to singlet oxygen / plant-type hypersensitive response / lipase activity / carboxylesterase / regulation of hydrogen peroxide metabolic process / carboxylesterase activity / lipid catabolic process / positive regulation of defense response to virus by host / chloroplast / lipid metabolic process / defense response to Gram-negative bacterium / response to hypoxia / endoplasmic reticulum / protein homodimerization activity / membrane / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.71 Å | |||||||||
 Authors Authors | Huang SJ / Jia AL / Han ZF / Chai JJ | |||||||||
| Funding support |  China, China,  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity. Authors: Aolin Jia / Shijia Huang / Wen Song / Junli Wang / Yonggang Meng / Yue Sun / Lina Xu / Henriette Laessle / Jan Jirschitzka / Jiao Hou / Tiantian Zhang / Wenquan Yu / Giuliana Hessler / ...Authors: Aolin Jia / Shijia Huang / Wen Song / Junli Wang / Yonggang Meng / Yue Sun / Lina Xu / Henriette Laessle / Jan Jirschitzka / Jiao Hou / Tiantian Zhang / Wenquan Yu / Giuliana Hessler / Ertong Li / Shoucai Ma / Dongli Yu / Jan Gebauer / Ulrich Baumann / Xiaohui Liu / Zhifu Han / Junbiao Chang / Jane E Parker / Jijie Chai /   Abstract: Plant pathogen-activated immune signaling by nucleotide-binding leucine-rich repeat (NLR) receptors with an N-terminal Toll/interleukin-1 receptor (TIR) domain converges on Enhanced Disease ...Plant pathogen-activated immune signaling by nucleotide-binding leucine-rich repeat (NLR) receptors with an N-terminal Toll/interleukin-1 receptor (TIR) domain converges on Enhanced Disease Susceptibility 1 (EDS1) and its direct partners, Phytoalexin Deficient 4 (PAD4) or Senescence-Associated Gene 101 (SAG101). TIR-encoded nicotinamide adenine dinucleotide hydrolase (NADase) produces signaling molecules to promote exclusive EDS1-PAD4 and EDS1-SAG101 interactions with helper NLR subclasses. In this work, we show that TIR-containing proteins catalyze adenosine diphosphate (ADP)-ribosylation of adenosine triphosphate (ATP) and ADP ribose (ADPR) through ADPR polymerase-like and NADase activity, forming ADP-ribosylated ATP (ADPr-ATP) and ADPr-ADPR (di-ADPR), respectively. Specific binding of ADPr-ATP or di-ADPR allosterically promotes EDS1-SAG101 interaction with helper NLR N requirement gene 1A (NRG1A) in vitro and in planta. Our data reveal an enzymatic activity of TIRs that enables specific activation of the EDS1-SAG101-NRG1 immunity branch. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33233.map.gz emd_33233.map.gz | 3.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33233-v30.xml emd-33233-v30.xml emd-33233.xml emd-33233.xml | 21.7 KB 21.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33233.png emd_33233.png | 22.7 KB | ||
| Filedesc metadata |  emd-33233.cif.gz emd-33233.cif.gz | 7.1 KB | ||
| Others |  emd_33233_half_map_1.map.gz emd_33233_half_map_1.map.gz emd_33233_half_map_2.map.gz emd_33233_half_map_2.map.gz | 40.8 MB 40.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33233 http://ftp.pdbj.org/pub/emdb/structures/EMD-33233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33233 | HTTPS FTP |
-Related structure data
| Related structure data |  7xjpMC  7xozC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33233.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33233.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0825 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33233_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
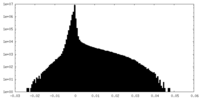
| Density Histograms |
-Half map: #1
| File | emd_33233_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
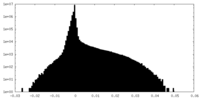
| Density Histograms |
- Sample components
Sample components
-Entire : Binary complex of EDS1 and SAG101 with molecule ATP-ADPR
| Entire | Name: Binary complex of EDS1 and SAG101 with molecule ATP-ADPR |
|---|---|
| Components |
|
-Supramolecule #1: Binary complex of EDS1 and SAG101 with molecule ATP-ADPR
| Supramolecule | Name: Binary complex of EDS1 and SAG101 with molecule ATP-ADPR type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Protein EDS1
| Macromolecule | Name: Protein EDS1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 71.784195 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAFEALTGIN GDLITRSWSA SKQAYLTERY HKEEAGAVVI FAFQPSFSEK DFFDPDNKSS FGEIKLNRVQ FPCMRKIGKG DVATVNEAF LKNLEAIIDP RTSFQASVEM AVRSRKQIVF TGHSSGGATA ILATVWYLEK YFIRNPNVYL EPRCVTFGAP L VGDSIFSH ...String: MAFEALTGIN GDLITRSWSA SKQAYLTERY HKEEAGAVVI FAFQPSFSEK DFFDPDNKSS FGEIKLNRVQ FPCMRKIGKG DVATVNEAF LKNLEAIIDP RTSFQASVEM AVRSRKQIVF TGHSSGGATA ILATVWYLEK YFIRNPNVYL EPRCVTFGAP L VGDSIFSH ALGREKWSRF FVNFVSRFDI VPRIMLARKA SVEETLPHVL AQLDPRKSSV QESEQRITEF YTRVMRDTST VA NQAVCEL TGSAEAFLET LSSFLELSPY RPAGTFVFST EKRLVAVNNS DAILQMLFYT SQASDEQEWS LIPFRSIRDH HSY EELVQS MGKKLFNHLD GENSIESTLN DLGVSTRGRQ YVQAALEEEK KRVENQKKII QVIEQERFLK KLAWIEDEYK PKCQ AHKNG YYDSFKVSNE ENDFKANVKR AELAGVFDEV LGLMKKCQLP DEFEGDIDWI KLATRYRRLV EPLDIANYHR HLKNE DTGP YMKRGRPTRY IYAQRGYEHY ILKPNGMIAE DVFWNKVNGL NLGLQLEEIQ ETLKNSGSEC GSCFWAEVEE LKGKPY EEV EVRVKTLEGM LGEWITDGEV DDKEIFLEGS TFRKWWITLP KNHKSHSPLR DYMMDEITDT UniProtKB: Protein EDS1 |
-Macromolecule #2: Senescence-associated carboxylesterase 101
| Macromolecule | Name: Senescence-associated carboxylesterase 101 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: carboxylesterase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 62.153391 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MESSSSLKGS ALGKLVVTSG LLHSSWSKIL EIHNPPYSNH DPGLQVSKKK KDSGLEFQIH REEKFTLVVF SAPPICRSSS SDSTLLHVK DKENPFPFLC SENNPSFSLH TPAFNLFTSA STSLTYLKSE LLQTLKSEKP VIITGAALGG SVASLYTLWL L ETIEPTLK ...String: MESSSSLKGS ALGKLVVTSG LLHSSWSKIL EIHNPPYSNH DPGLQVSKKK KDSGLEFQIH REEKFTLVVF SAPPICRSSS SDSTLLHVK DKENPFPFLC SENNPSFSLH TPAFNLFTSA STSLTYLKSE LLQTLKSEKP VIITGAALGG SVASLYTLWL L ETIEPTLK RPLCITFGSP LIGDASLQQI LENSVRNSCF LHVVSAQTRI KMDFFKPFGT FLICFDSGCV CIEDHVAVTE LL NGVHDSG LVDYSQVLNR LDQSMLSLAD SRLIPEDVIK GIEKRAEMKN LRFDMMFKKL NDMKISMAYI EWYKKKCKEV KIG YYDRFK TQLAFPSKEF DINIKNHHKS ELNRFWKSVV EEVERRPQSD ASILKRRFLF SGNNYRRMIE PLDIAEYYLE GRKE YRTTG RSHHYVMLEK WFGMESILIE KERCKKRDLS DLLTFDSCFW AEVEDSLIVI NQLNTTVGMR DDVREVLTRK LVEFE GYVW EIITKREVSP EIFLEESSFM KWWKEYKKIK GFNSSYLTEF MNTRKYESYG KSQ UniProtKB: Senescence-associated carboxylesterase 101 |
-Macromolecule #3: ISOPROPYL ALCOHOL
| Macromolecule | Name: ISOPROPYL ALCOHOL / type: ligand / ID: 3 / Number of copies: 1 / Formula: IPA |
|---|---|
| Molecular weight | Theoretical: 60.095 Da |
| Chemical component information |  ChemComp-IPA: |
-Macromolecule #4: ADENOSINE-5-DIPHOSPHORIBOSE
| Macromolecule | Name: ADENOSINE-5-DIPHOSPHORIBOSE / type: ligand / ID: 4 / Number of copies: 1 / Formula: APR |
|---|---|
| Molecular weight | Theoretical: 559.316 Da |
| Chemical component information | 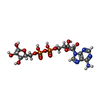 ChemComp-APR: |
-Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)