[English] 日本語
 Yorodumi
Yorodumi- EMDB-33108: Cryo-EM structure of the human chemokine receptor CX3CR1 in compl... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human chemokine receptor CX3CR1 in complex with CX3CL1 and Gi1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | G protein-coupled receptor / chemokine receptor / CX3CR1 / CX3CL1 / SIGNALING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationC-X3-C chemokine receptor activity / dendritic tree / multiple spine synapse organization, single dendrite / negative regulation of microglial cell mediated cytotoxicity / macropinosome membrane / C-X3-C chemokine binding / regulation of microglial cell migration / CXCR1 chemokine receptor binding / positive regulation of calcium-independent cell-cell adhesion / negative regulation of interleukin-1 alpha production ...C-X3-C chemokine receptor activity / dendritic tree / multiple spine synapse organization, single dendrite / negative regulation of microglial cell mediated cytotoxicity / macropinosome membrane / C-X3-C chemokine binding / regulation of microglial cell migration / CXCR1 chemokine receptor binding / positive regulation of calcium-independent cell-cell adhesion / negative regulation of interleukin-1 alpha production / leukocyte adhesive activation / CX3C chemokine receptor binding / negative regulation of glutamate receptor signaling pathway / microglial cell activation involved in immune response / autocrine signaling / lymphocyte chemotaxis / host-mediated modulation of intestinal microbiota composition / synapse pruning / positive regulation of microglial cell migration / central nervous system maturation / regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of microglial cell activation / synapse maturation / negative regulation of neuron migration / negative regulation of hippocampal neuron apoptotic process / antifungal innate immune response / positive regulation of transforming growth factor beta1 production / chemokine receptor activity / CCR chemokine receptor binding / microglial cell proliferation / positive regulation of actin filament bundle assembly / leukocyte migration involved in inflammatory response / leukocyte tethering or rolling / C-C chemokine receptor activity / integrin activation / regulation of tumor necrosis factor production / chemokine-mediated signaling pathway / C-C chemokine binding / eosinophil chemotaxis / positive regulation of monocyte chemotaxis / leukocyte chemotaxis / G protein-coupled peptide receptor activity / regulation of nitric oxide biosynthetic process / angiogenesis involved in wound healing / chemokine activity / positive regulation of neurogenesis / Chemokine receptors bind chemokines / negative regulation of interleukin-1 beta production / positive regulation of cell-matrix adhesion / neuronal cell body membrane / neuron remodeling / positive regulation of neuroblast proliferation / positive chemotaxis / RSV-host interactions / chemoattractant activity / macrophage chemotaxis / negative regulation of interleukin-6 production / Respiratory syncytial virus (RSV) attachment and entry / negative regulation of apoptotic signaling pathway / negative regulation of tumor necrosis factor production / social behavior / negative regulation of cell-substrate adhesion / regulation of neurogenesis / negative regulation of extrinsic apoptotic signaling pathway in absence of ligand / cellular defense response / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / extrinsic apoptotic signaling pathway in absence of ligand / neutrophil chemotaxis / positive regulation of smooth muscle cell proliferation / cellular response to forskolin / regulation of mitotic spindle organization / negative regulation of angiogenesis / positive regulation of release of sequestered calcium ion into cytosol / negative regulation of cell migration / response to ischemia / cell chemotaxis / cell projection / Regulation of insulin secretion / microglial cell activation / calcium-mediated signaling / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor activity / G protein-coupled receptor binding / defense response / cell-cell adhesion / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / positive regulation of neuron projection development / modulation of chemical synaptic transmission / neuron cellular homeostasis / brain development Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Lu M / Zhao W / Han S / Zhu Y / Wu B / Zhao Q | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Activation of the human chemokine receptor CX3CR1 regulated by cholesterol. Authors: Minmin Lu / Wenli Zhao / Shuo Han / Xiaowen Lin / Tingyu Xu / Qiuxiang Tan / Mu Wang / Cuiying Yi / Xiaojing Chu / Weibo Yang / Ya Zhu / Beili Wu / Qiang Zhao /  Abstract: As the only member of the CX3C chemokine receptor subfamily, CX3CR1 binds to its sole endogenous ligand CX3CL1, which shows notable potential as a therapeutic target in atherosclerosis, cancer, and ...As the only member of the CX3C chemokine receptor subfamily, CX3CR1 binds to its sole endogenous ligand CX3CL1, which shows notable potential as a therapeutic target in atherosclerosis, cancer, and neuropathy. However, the drug development of CX3CR1 is hampered partially by the lack of structural information. Here, we present two cryo-electron microscopy structures of CX3CR1-G complexes in ligand-free and CX3CL1-bound states at 2.8- and 3.4-Å resolution, respectively. Together with functional data, the structures reveal the key factors that govern the recognition of CX3CL1 by both CX3CR1 and US28. A much smaller conformational change of helix VI upon activation than previously solved class A GPCR-G complex structures is observed in CX3CR1, which may correlate with three cholesterol molecules that play essential roles in conformation stabilization and signaling transduction. Thus, our data deepen the understanding of cholesterol modulation in GPCR (G protein-coupled receptor) signaling and provide insights into the diversity of G protein coupling. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33108.map.gz emd_33108.map.gz | 59.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33108-v30.xml emd-33108-v30.xml emd-33108.xml emd-33108.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33108.png emd_33108.png | 95.3 KB | ||
| Filedesc metadata |  emd-33108.cif.gz emd-33108.cif.gz | 6.3 KB | ||
| Others |  emd_33108_half_map_1.map.gz emd_33108_half_map_1.map.gz emd_33108_half_map_2.map.gz emd_33108_half_map_2.map.gz | 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33108 http://ftp.pdbj.org/pub/emdb/structures/EMD-33108 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33108 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33108 | HTTPS FTP |
-Validation report
| Summary document |  emd_33108_validation.pdf.gz emd_33108_validation.pdf.gz | 810.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33108_full_validation.pdf.gz emd_33108_full_validation.pdf.gz | 810.4 KB | Display | |
| Data in XML |  emd_33108_validation.xml.gz emd_33108_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_33108_validation.cif.gz emd_33108_validation.cif.gz | 14.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33108 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33108 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33108 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33108 | HTTPS FTP |
-Related structure data
| Related structure data |  7xbxMC  7xbwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33108.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33108.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.045 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33108_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
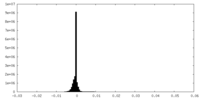
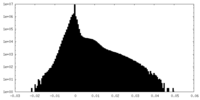
| Density Histograms |
-Half map: #2
| File | emd_33108_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
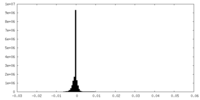
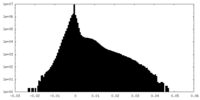
| Density Histograms |
- Sample components
Sample components
-Entire : Chemokine receptor CX3CR1 in complex with CX3CL1 Gi1
| Entire | Name: Chemokine receptor CX3CR1 in complex with CX3CL1 Gi1 |
|---|---|
| Components |
|
-Supramolecule #1: Chemokine receptor CX3CR1 in complex with CX3CL1 Gi1
| Supramolecule | Name: Chemokine receptor CX3CR1 in complex with CX3CL1 Gi1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.447141 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKCTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKCTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVTAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.245805 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HHHHHHMSEL DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSAS QDGKLIIWD SYTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT GYLSCCRFLD D NQIVTSSG ...String: HHHHHHMSEL DQLRQEAEQL KNQIRDARKA CADATLSQIT NNIDPVGRIQ MRTRRTLRGH LAKIYAMHWG TDSRLLVSAS QDGKLIIWD SYTTNKVHAI PLRSSWVMTC AYAPSGNYVA CGGLDNICSI YNLKTREGNV RVSRELAGHT GYLSCCRFLD D NQIVTSSG DTTCALWDIE TGQQTTTFTG HTGDVMSLSL APDTRLFVSG ACDASAKLWD VREGMCRQTF TGHESDINAI CF FPNGNAF ATGSDDATCR LFDLRADQEL MTYSHDNIIC GITSVSFSKS GRLLLAGYDD FNCNVWDALK ADRAGVLAGH DNR VSCLGV TDDGMAVATG SWDSFLKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Processed fractalkine,CX3C chemokine receptor 1
| Macromolecule | Name: Processed fractalkine,CX3C chemokine receptor 1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.55825 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: (PCA)HHGVTKCNI TCSKMTSKIP VALLIHYQQN QASCCKRAII LETRQHRLFC ADPKEQWVKD AMQHLDRQAA ALTRNG SGS GSGSGSGSGS GSGSGSGSGS GSGSDQFPES VTENFEYDDL AEACYIGDIV VFGTVFLSIF YSVIFAIGLV GNLLVVF AL TNSKKPKSVT ...String: (PCA)HHGVTKCNI TCSKMTSKIP VALLIHYQQN QASCCKRAII LETRQHRLFC ADPKEQWVKD AMQHLDRQAA ALTRNG SGS GSGSGSGSGS GSGSGSGSGS GSGSDQFPES VTENFEYDDL AEACYIGDIV VFGTVFLSIF YSVIFAIGLV GNLLVVF AL TNSKKPKSVT DIYLLNLALS DLLFVATLPF WTHYLINEKG LHNAMCKFTT AFFFIGFFGS IFFLTVISID RYLAIVLA A NSMNNRTVQH GVTISLGVWA AAILVAAPQF MFTKQKENEC CGDYPEVLQE IWPVLRNVET NFLGFLLPLL IMSYCYFRI IQTLFSSKNH KKAKAIKLIL LVVIVFFLFW TPYNVVIFLE TLKLYDFFPS CDMRKDLRLA LSVTETVAFS HCCLNPLIYA FAGEKFRRY LYHLYGKCLA VLEFLEVLFQ GPWSHPQFEK GGGSGGGSGG SAWSHPQFEK DYKDDDDK UniProtKB: Fractalkine, CX3C chemokine receptor 1 |
-Macromolecule #5: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 5 / Number of copies: 2 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 2.1875 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 490779 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)