[English] 日本語
 Yorodumi
Yorodumi- EMDB-33049: Cryo-EM structure of SARS-CoV spike protein in complex with three... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of SARS-CoV spike protein in complex with three nAbs X01, X10 and X17 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Neutralizing antibody / Cryo-EM / VIRAL PROTEIN / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell ...Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |   Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.83 Å | |||||||||
 Authors Authors | Sun H / Liu L / Zhang T / Zheng Q / Li S / Xia N | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: The neutralizing breadth of antibodies targeting diverse conserved epitopes between SARS-CoV and SARS-CoV-2. Authors: Hualong Xiong / Hui Sun / Siling Wang / Lunzhi Yuan / Liqin Liu / Yuhe Zhu / Jinlei Zhang / Yang Huang / Ruoyao Qi / Yao Jiang / Jian Ma / Ming Zhou / Yue Ma / Rao Fu / Siping Yan / Mingxi ...Authors: Hualong Xiong / Hui Sun / Siling Wang / Lunzhi Yuan / Liqin Liu / Yuhe Zhu / Jinlei Zhang / Yang Huang / Ruoyao Qi / Yao Jiang / Jian Ma / Ming Zhou / Yue Ma / Rao Fu / Siping Yan / Mingxi Yue / Yangtao Wu / Min Wei / Yizhen Wang / Tingting Li / Yingbin Wang / Zizheng Zheng / Hai Yu / Tong Cheng / Shaowei Li / Quan Yuan / Jun Zhang / Yi Guan / Qingbing Zheng / Tianying Zhang / Ningshao Xia /  Abstract: Antibody therapeutics for the treatment of COVID-19 have been highly successful. However, the recent emergence of the Omicron variant has posed a challenge, as it evades detection by most existing ...Antibody therapeutics for the treatment of COVID-19 have been highly successful. However, the recent emergence of the Omicron variant has posed a challenge, as it evades detection by most existing SARS-CoV-2 neutralizing antibodies (nAbs). Here, we successfully generated a panel of SARS-CoV-2/SARS-CoV cross-neutralizing antibodies by sequential immunization of the two pseudoviruses. Of the potential candidates, we found that nAbs X01, X10, and X17 offer broad neutralizing potential against most variants of concern, with X17 further identified as a Class 5 nAb with undiminished neutralization against the Omicron variant. Cryo-electron microscopy structures of the three antibodies together in complex with each of the spike proteins of the prototypical SARS-CoV, SARS-CoV-2, and Delta and Omicron variants of SARS-CoV-2 defined three nonoverlapping conserved epitopes on the receptor-binding domain. The triple-antibody mixture exhibited enhanced resistance to viral evasion and effective protection against infection of the Beta variant in hamsters. Our findings will aid the development of antibody therapeutics and broad vaccines against SARS-CoV-2 and its emerging variants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33049.map.gz emd_33049.map.gz | 684.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33049-v30.xml emd-33049-v30.xml emd-33049.xml emd-33049.xml | 17.3 KB 17.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33049.png emd_33049.png | 20 KB | ||
| Filedesc metadata |  emd-33049.cif.gz emd-33049.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33049 http://ftp.pdbj.org/pub/emdb/structures/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33049 | HTTPS FTP |
-Validation report
| Summary document |  emd_33049_validation.pdf.gz emd_33049_validation.pdf.gz | 510.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33049_full_validation.pdf.gz emd_33049_full_validation.pdf.gz | 509.8 KB | Display | |
| Data in XML |  emd_33049_validation.xml.gz emd_33049_validation.xml.gz | 8.3 KB | Display | |
| Data in CIF |  emd_33049_validation.cif.gz emd_33049_validation.cif.gz | 9.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33049 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33049 | HTTPS FTP |
-Related structure data
| Related structure data |  7x7vMC  7x7tC  7x7uC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33049.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33049.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.778 Å | ||||||||||||||||||||||||||||||||||||
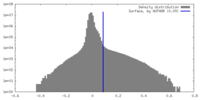
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Cryo-EM structure of SARS-CoV spike protein in complex with three...
+Supramolecule #1: Cryo-EM structure of SARS-CoV spike protein in complex with three...
+Supramolecule #2: nAbs X01, X10 and X17
+Supramolecule #3: SARS-CoV spike protein
+Macromolecule #1: X10 light chain
+Macromolecule #2: X10 heavy chain
+Macromolecule #3: X17 light chain
+Macromolecule #4: X17 heavy chain
+Macromolecule #5: X01 light chain
+Macromolecule #6: X01 heavy chain
+Macromolecule #7: Spike protein S1
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.83 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 230838 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















 Homo sapiens (human)
Homo sapiens (human)