+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
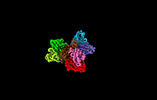
| タイトル | Cryo-EM structure of SARS-CoV spike protein in complex with three nAbs X01, X10 and X17 | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | SARS-CoV-2 / Neutralizing antibody / Cryo-EM / VIRAL PROTEIN / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell ...Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / SARS-CoV-1 activates/modulates innate immune responses / symbiont-mediated-mediated suppression of host tetherin activity / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane / identical protein binding / membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |   Severe acute respiratory syndrome coronavirus (SARS コロナウイルス) Severe acute respiratory syndrome coronavirus (SARS コロナウイルス) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.83 Å | |||||||||
 データ登録者 データ登録者 | Sun H / Liu L / Zhang T / Zheng Q / Li S / Xia N | |||||||||
| 資金援助 | 1件
| |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2022 ジャーナル: Proc Natl Acad Sci U S A / 年: 2022タイトル: The neutralizing breadth of antibodies targeting diverse conserved epitopes between SARS-CoV and SARS-CoV-2. 著者: Hualong Xiong / Hui Sun / Siling Wang / Lunzhi Yuan / Liqin Liu / Yuhe Zhu / Jinlei Zhang / Yang Huang / Ruoyao Qi / Yao Jiang / Jian Ma / Ming Zhou / Yue Ma / Rao Fu / Siping Yan / Mingxi ...著者: Hualong Xiong / Hui Sun / Siling Wang / Lunzhi Yuan / Liqin Liu / Yuhe Zhu / Jinlei Zhang / Yang Huang / Ruoyao Qi / Yao Jiang / Jian Ma / Ming Zhou / Yue Ma / Rao Fu / Siping Yan / Mingxi Yue / Yangtao Wu / Min Wei / Yizhen Wang / Tingting Li / Yingbin Wang / Zizheng Zheng / Hai Yu / Tong Cheng / Shaowei Li / Quan Yuan / Jun Zhang / Yi Guan / Qingbing Zheng / Tianying Zhang / Ningshao Xia /  要旨: Antibody therapeutics for the treatment of COVID-19 have been highly successful. However, the recent emergence of the Omicron variant has posed a challenge, as it evades detection by most existing ...Antibody therapeutics for the treatment of COVID-19 have been highly successful. However, the recent emergence of the Omicron variant has posed a challenge, as it evades detection by most existing SARS-CoV-2 neutralizing antibodies (nAbs). Here, we successfully generated a panel of SARS-CoV-2/SARS-CoV cross-neutralizing antibodies by sequential immunization of the two pseudoviruses. Of the potential candidates, we found that nAbs X01, X10, and X17 offer broad neutralizing potential against most variants of concern, with X17 further identified as a Class 5 nAb with undiminished neutralization against the Omicron variant. Cryo-electron microscopy structures of the three antibodies together in complex with each of the spike proteins of the prototypical SARS-CoV, SARS-CoV-2, and Delta and Omicron variants of SARS-CoV-2 defined three nonoverlapping conserved epitopes on the receptor-binding domain. The triple-antibody mixture exhibited enhanced resistance to viral evasion and effective protection against infection of the Beta variant in hamsters. Our findings will aid the development of antibody therapeutics and broad vaccines against SARS-CoV-2 and its emerging variants. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_33049.map.gz emd_33049.map.gz | 684.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-33049-v30.xml emd-33049-v30.xml emd-33049.xml emd-33049.xml | 17.3 KB 17.3 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_33049.png emd_33049.png | 20 KB | ||
| Filedesc metadata |  emd-33049.cif.gz emd-33049.cif.gz | 6.1 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33049 http://ftp.pdbj.org/pub/emdb/structures/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33049 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7x7vMC  7x7tC  7x7uC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_33049.map.gz / 形式: CCP4 / 大きさ: 729 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_33049.map.gz / 形式: CCP4 / 大きさ: 729 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.778 Å | ||||||||||||||||||||||||||||||||||||
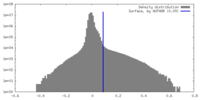
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : Cryo-EM structure of SARS-CoV spike protein in complex with three...
+超分子 #1: Cryo-EM structure of SARS-CoV spike protein in complex with three...
+超分子 #2: nAbs X01, X10 and X17
+超分子 #3: SARS-CoV spike protein
+分子 #1: X10 light chain
+分子 #2: X10 heavy chain
+分子 #3: X17 light chain
+分子 #4: X17 heavy chain
+分子 #5: X01 light chain
+分子 #6: X01 heavy chain
+分子 #7: Spike protein S1
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F30 |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 60.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 0.8 µm |
| 実験機器 |  モデル: Tecnai F30 / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: OTHER |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.83 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 230838 |
| 初期 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
 ムービー
ムービー コントローラー
コントローラー










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















 Homo sapiens (ヒト)
Homo sapiens (ヒト)