+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of the tavapadon-bound D1 dopamine receptor and mini-Gs complex | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | GPCR / dopamine receptor / mini-Gs / MEMBRANE PROTEIN / tavapadon | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報dopamine neurotransmitter receptor activity, coupled via Gs / dopamine neurotransmitter receptor activity / operant conditioning / cerebral cortex GABAergic interneuron migration / Dopamine receptors / dopamine binding / regulation of dopamine uptake involved in synaptic transmission / phospholipase C-activating dopamine receptor signaling pathway / peristalsis / heterotrimeric G-protein binding ...dopamine neurotransmitter receptor activity, coupled via Gs / dopamine neurotransmitter receptor activity / operant conditioning / cerebral cortex GABAergic interneuron migration / Dopamine receptors / dopamine binding / regulation of dopamine uptake involved in synaptic transmission / phospholipase C-activating dopamine receptor signaling pathway / peristalsis / heterotrimeric G-protein binding / modification of postsynaptic structure / G protein-coupled receptor complex / regulation of dopamine metabolic process / positive regulation of neuron migration / grooming behavior / habituation / sensitization / dopamine transport / astrocyte development / dentate gyrus development / conditioned taste aversion / striatum development / positive regulation of potassium ion transport / maternal behavior / arrestin family protein binding / non-motile cilium / long-term synaptic depression / mating behavior / adult walking behavior / G protein-coupled dopamine receptor signaling pathway / ciliary membrane / temperature homeostasis / D-glucose import / dopamine metabolic process / transmission of nerve impulse / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / G-protein alpha-subunit binding / positive regulation of synaptic transmission, glutamatergic / behavioral fear response / prepulse inhibition / neuronal action potential / behavioral response to cocaine / synapse assembly / adenylate cyclase-activating adrenergic receptor signaling pathway / presynaptic modulation of chemical synaptic transmission / positive regulation of release of sequestered calcium ion into cytosol / response to amphetamine / synaptic transmission, glutamatergic / G protein-coupled receptor activity / visual learning / GABA-ergic synapse / Olfactory Signaling Pathway / vasodilation / memory / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / long-term synaptic potentiation / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / protein import into nucleus / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / cellular response to catecholamine stimulus / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / presynaptic membrane / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / synthetic construct (人工物) Homo sapiens (ヒト) / synthetic construct (人工物) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.3 Å | |||||||||
 データ登録者 データ登録者 | Teng X / Zheng S | |||||||||
| 資金援助 |  中国, 1件 中国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2022 ジャーナル: Nat Commun / 年: 2022タイトル: Ligand recognition and biased agonism of the D1 dopamine receptor. 著者: Xiao Teng / Sijia Chen / Yingying Nie / Peng Xiao / Xiao Yu / Zhenhua Shao / Sanduo Zheng /  要旨: Dopamine receptors are widely distributed in the central nervous system and are important therapeutic targets for treatment of various psychiatric and neurological diseases. Here, we report three ...Dopamine receptors are widely distributed in the central nervous system and are important therapeutic targets for treatment of various psychiatric and neurological diseases. Here, we report three cryo-electron microscopy structures of the D1 dopamine receptor (D1R)-Gs complex bound to two agonists, fenoldopam and tavapadon, and a positive allosteric modulator LY3154207. The structure reveals unusual binding of two fenoldopam molecules, one to the orthosteric binding pocket (OBP) and the other to the extended binding pocket (EBP). In contrast, one elongated tavapadon molecule binds to D1R, extending from OBP to EBP. Moreover, LY3154207 stabilizes the second intracellular loop of D1R in an alpha helical conformation to efficiently engage the G protein. Through a combination of biochemical, biophysical and cellular assays, we further show that the broad conformation stabilized by two fenoldopam molecules and interaction between TM5 and the agonist are important for biased signaling of D1R. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_32965.map.gz emd_32965.map.gz | 20.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-32965-v30.xml emd-32965-v30.xml emd-32965.xml emd-32965.xml | 21 KB 21 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_32965.png emd_32965.png | 32.1 KB | ||
| Filedesc metadata |  emd-32965.cif.gz emd-32965.cif.gz | 7.3 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32965 http://ftp.pdbj.org/pub/emdb/structures/EMD-32965 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32965 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32965 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_32965_validation.pdf.gz emd_32965_validation.pdf.gz | 494.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_32965_full_validation.pdf.gz emd_32965_full_validation.pdf.gz | 494.1 KB | 表示 | |
| XML形式データ |  emd_32965_validation.xml.gz emd_32965_validation.xml.gz | 5.7 KB | 表示 | |
| CIF形式データ |  emd_32965_validation.cif.gz emd_32965_validation.cif.gz | 6.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32965 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32965 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32965 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32965 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_32965.map.gz / 形式: CCP4 / 大きさ: 22.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_32965.map.gz / 形式: CCP4 / 大きさ: 22.2 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.087 Å | ||||||||||||||||||||||||||||||||||||
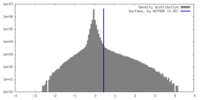
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : Cryo-EM structure of the tavapadon-bound D1R-miniGs complex
+超分子 #1: Cryo-EM structure of the tavapadon-bound D1R-miniGs complex
+超分子 #2: tavapadon-bound D1R-miniGs
+超分子 #3: nanobody35
+分子 #1: D(1A) dopamine receptor
+分子 #2: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
+分子 #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+分子 #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+分子 #5: Nanobody35
+分子 #6: CHOLESTEROL
+分子 #7: 1,5-dimethyl-6-[2-methyl-4-[3-(trifluoromethyl)pyridin-2-yl]oxy-p...
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 4.0 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: GOLD / メッシュ: 300 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 35 sec. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 281 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 実像数: 1861 / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.0 µm 最小 デフォーカス(公称値): 0.7000000000000001 µm 倍率(公称値): 64000 |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















 Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ)