+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
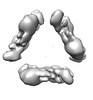
| Title | CryoEM structure of sNS1 hexamer | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NS1 protein / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host JAK-STAT cascade via inhibition of host TYK2 activity / host cell mitochondrion / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT2 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / ribonucleoside triphosphate phosphatase activity / viral capsid / double-stranded RNA binding / channel activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell ...symbiont-mediated suppression of host JAK-STAT cascade via inhibition of host TYK2 activity / host cell mitochondrion / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT2 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / ribonucleoside triphosphate phosphatase activity / viral capsid / double-stranded RNA binding / channel activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / methyltransferase cap1 activity / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / RNA helicase activity / protein dimerization activity / host cell endoplasmic reticulum membrane / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / symbiont-mediated activation of host autophagy / serine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell nucleus / virion membrane / structural molecule activity / proteolysis / extracellular region / ATP binding / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Dengue virus 2 Dengue virus 2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 8.0 Å | |||||||||
 Authors Authors | Shu B / Ooi JSG | |||||||||
| Funding support |  Singapore, 1 items Singapore, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever. Authors: Bo Shu / Justin S G Ooi / Aaron W K Tan / Thiam-Seng Ng / Wanwisa Dejnirattisai / Juthathip Mongkolsapaya / Guntur Fibriansah / Jian Shi / Victor A Kostyuchenko / Gavin R Screaton / Shee-Mei Lok /   Abstract: Dengue virus infection can cause dengue hemorrhagic fever (DHF). Dengue NS1 is multifunctional. The intracellular dimeric NS1 (iNS1) forms part of the viral replication complex. Previous studies ...Dengue virus infection can cause dengue hemorrhagic fever (DHF). Dengue NS1 is multifunctional. The intracellular dimeric NS1 (iNS1) forms part of the viral replication complex. Previous studies suggest the extracellular secreted NS1 (sNS1), which is a major factor contributing to DHF, exists as hexamers. The structure of the iNS1 is well-characterised but not that of sNS1. Here we show by cryoEM that the recombinant sNS1 exists in multiple oligomeric states: the tetrameric (stable and loose conformation) and hexameric structures. Stability of the stable and loose tetramers is determined by the conformation of their N-terminal domain - elongated β-sheet or β-roll. Binding of an anti-NS1 Fab breaks the loose tetrameric and hexameric sNS1 into dimers, whereas the stable tetramer remains largely unbound. Our results show detailed quaternary organization of different oligomeric states of sNS1 and will contribute towards the design of dengue therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32843.map.gz emd_32843.map.gz | 11.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32843-v30.xml emd-32843-v30.xml emd-32843.xml emd-32843.xml | 10.1 KB 10.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32843.png emd_32843.png | 51.8 KB | ||
| Filedesc metadata |  emd-32843.cif.gz emd-32843.cif.gz | 5.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32843 http://ftp.pdbj.org/pub/emdb/structures/EMD-32843 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32843 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32843 | HTTPS FTP |
-Validation report
| Summary document |  emd_32843_validation.pdf.gz emd_32843_validation.pdf.gz | 412.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32843_full_validation.pdf.gz emd_32843_full_validation.pdf.gz | 412.3 KB | Display | |
| Data in XML |  emd_32843_validation.xml.gz emd_32843_validation.xml.gz | 5.8 KB | Display | |
| Data in CIF |  emd_32843_validation.cif.gz emd_32843_validation.cif.gz | 6.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32843 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32843 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32843 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32843 | HTTPS FTP |
-Related structure data
| Related structure data |  7wuvMC  7wurC  7wusC  7wutC  7wuuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32843.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32843.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.342 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : NS1 Hexamer
| Entire | Name: NS1 Hexamer |
|---|---|
| Components |
|
-Supramolecule #1: NS1 Hexamer
| Supramolecule | Name: NS1 Hexamer / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Dengue virus 2 Dengue virus 2 |
-Macromolecule #1: Core protein
| Macromolecule | Name: Core protein / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: flavivirin |
|---|---|
| Source (natural) | Organism:  Dengue virus 2 Dengue virus 2 |
| Molecular weight | Theoretical: 34.838719 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: PESPSKLASA IQKAHEEGIC GIRSVTRLEN LMWKQITPEL NHILTENEVK LTIMTGDIKG IMQAGKRSLR PQPTELKYSW KAWGKAKML STELHNHTFL IDGPETAECP NTNRAWNSLE VEDYGFGVFT TNIWLKLKER QDVFCDSKLM SAAIKDNRAV H ADMGYWIE ...String: PESPSKLASA IQKAHEEGIC GIRSVTRLEN LMWKQITPEL NHILTENEVK LTIMTGDIKG IMQAGKRSLR PQPTELKYSW KAWGKAKML STELHNHTFL IDGPETAECP NTNRAWNSLE VEDYGFGVFT TNIWLKLKER QDVFCDSKLM SAAIKDNRAV H ADMGYWIE SALNDTWKIE KASFIEVKSC HWPKSHTLWS NGVLESEMII PKNFAGPVSQ HNYRPGYHTQ TAGPWHLGRL EM DFDFCEG TTVVVTEDCG NRGPSLRTTT ASGKLITEWC CRSCTLPPLR YRGEDGCWYG MEIRPLKEK UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 81.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 8.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 37422 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















