+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human SGLT1-MAP17 complex bound with LX2761 | ||||||||||||
 Map data Map data | Sharpened map of human SGLT1-MAP17 complex with LX2761. | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | glucose transporter / SGLT / sodium glucose transporter / membrane protein / PROTEIN TRANSPORT | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmyo-inositol:sodium symporter activity / pentose transmembrane transporter activity / galactose:sodium symporter activity / pentose transmembrane transport / myo-inositol transport / intestinal hexose absorption / Defective SLC5A1 causes congenital glucose/galactose malabsorption (GGM) / Intestinal hexose absorption / fucose transmembrane transport / fucose transmembrane transporter activity ...myo-inositol:sodium symporter activity / pentose transmembrane transporter activity / galactose:sodium symporter activity / pentose transmembrane transport / myo-inositol transport / intestinal hexose absorption / Defective SLC5A1 causes congenital glucose/galactose malabsorption (GGM) / Intestinal hexose absorption / fucose transmembrane transport / fucose transmembrane transporter activity / intestinal D-glucose absorption / galactose transmembrane transporter activity / alpha-glucoside transport / alpha-glucoside transmembrane transporter activity / D-glucose:sodium symporter activity / galactose transmembrane transport / renal D-glucose absorption / water transmembrane transporter activity / D-glucose import across plasma membrane / Cellular hexose transport / D-glucose transmembrane transporter activity / D-glucose transmembrane transport / transepithelial water transport / sodium ion import across plasma membrane / sodium ion transport / intracellular vesicle / transport across blood-brain barrier / brush border membrane / nuclear membrane / early endosome / apical plasma membrane / perinuclear region of cytoplasm / Golgi apparatus / extracellular exosome / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Chen L / Niu Y / Cui W | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural mechanism of SGLT1 inhibitors. Authors: Yange Niu / Wenhao Cui / Rui Liu / Sanshan Wang / Han Ke / Xiaoguang Lei / Lei Chen /  Abstract: Sodium glucose co-transporters (SGLT) harness the electrochemical gradient of sodium to drive the uphill transport of glucose across the plasma membrane. Human SGLT1 (hSGLT1) plays a key role in ...Sodium glucose co-transporters (SGLT) harness the electrochemical gradient of sodium to drive the uphill transport of glucose across the plasma membrane. Human SGLT1 (hSGLT1) plays a key role in sugar uptake from food and its inhibitors show promise in the treatment of several diseases. However, the inhibition mechanism for hSGLT1 remains elusive. Here, we present the cryo-EM structure of the hSGLT1-MAP17 hetero-dimeric complex in the presence of the high-affinity inhibitor LX2761. LX2761 locks the transporter in an outward-open conformation by wedging inside the substrate-binding site and the extracellular vestibule of hSGLT1. LX2761 blocks the putative water permeation pathway of hSGLT1. The structure also uncovers the conformational changes of hSGLT1 during transitions from outward-open to inward-open states. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32617.map.gz emd_32617.map.gz | 79 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32617-v30.xml emd-32617-v30.xml emd-32617.xml emd-32617.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32617.png emd_32617.png | 105.9 KB | ||
| Filedesc metadata |  emd-32617.cif.gz emd-32617.cif.gz | 5.7 KB | ||
| Others |  emd_32617_half_map_1.map.gz emd_32617_half_map_1.map.gz emd_32617_half_map_2.map.gz emd_32617_half_map_2.map.gz | 77.6 MB 77.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32617 http://ftp.pdbj.org/pub/emdb/structures/EMD-32617 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32617 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32617 | HTTPS FTP |
-Validation report
| Summary document |  emd_32617_validation.pdf.gz emd_32617_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32617_full_validation.pdf.gz emd_32617_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_32617_validation.xml.gz emd_32617_validation.xml.gz | 13 KB | Display | |
| Data in CIF |  emd_32617_validation.cif.gz emd_32617_validation.cif.gz | 15.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32617 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32617 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32617 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32617 | HTTPS FTP |
-Related structure data
| Related structure data |  7wmvMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32617.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32617.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of human SGLT1-MAP17 complex with LX2761. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.821 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : human SGLT1-MAP17 complex
| Entire | Name: human SGLT1-MAP17 complex |
|---|---|
| Components |
|
-Supramolecule #1: human SGLT1-MAP17 complex
| Supramolecule | Name: human SGLT1-MAP17 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sodium/glucose cotransporter 1
| Macromolecule | Name: Sodium/glucose cotransporter 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 73.557703 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDSSTWSPKT TAVTRPVETH ELIRNAADIS IIVIYFVVVM AVGLWAMFST NRGTVGGFFL AGRSMVWWPI GASLFASNIG SGHFVGLAG TGAASGIAIG GFEWNALVLV VVLGWLFVPI YIKAGVVTMP EYLRKRFGGQ RIQVYLSLLS LLLYIFTKIS A DIFSGAIF ...String: MDSSTWSPKT TAVTRPVETH ELIRNAADIS IIVIYFVVVM AVGLWAMFST NRGTVGGFFL AGRSMVWWPI GASLFASNIG SGHFVGLAG TGAASGIAIG GFEWNALVLV VVLGWLFVPI YIKAGVVTMP EYLRKRFGGQ RIQVYLSLLS LLLYIFTKIS A DIFSGAIF INLALGLNLY LAIFLLLAIT ALYTITGGLA AVIYTDTLQT VIMLVGSLIL TGFAFHEVGG YDAFMEKYMK AI PTIVSDG NTTFQEKCYT PRADSFHIFR DPLTGDLPWP GFIFGMSILT LWYWCTDQVI VQRCLSAKNM SHVKGGCILC GYL KLMPMF IMVMPGMISR ILYTEKIACV VPSECEKYCG TKVGCTNIAY PTLVVELMPN GLRGLMLSVM LASLMSSLTS IFNS ASTLF TMDIYAKVRK RASEKELMIA GRLFILVLIG ISIAWVPIVQ SAQSGQLFDY IQSITSYLGP PIAAVFLLAI FWKRV NEPG AFWGLILGLL IGISRMITEF AYGTGSCMEP SNCPTIICGV HYLYFAIILF AISFITIVVI SLLTKPIPDV HLYRLC WSL RNSKEERIDL DAEEENIQEG PKETIEIETQ VPEKKKGIFR RAYDLFCGLE QHGAPKMTEE EEKAMKMKMT DTSEKPL WR TVLNVNGIIL VTVAVFCHAY FA UniProtKB: Sodium/glucose cotransporter 1 |
-Macromolecule #2: PDZK1-interacting protein 1
| Macromolecule | Name: PDZK1-interacting protein 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.265092 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSALSLLILG LLMAVPPASC QQGLGNLQPW MQGLIAVAVF LVLVAIAFAV NHFWCQEEPE PAHMILTVGN KADGVLVGTD GRYSSMAAS FRSSEHENAY ENVPEEEGKV RSTPM UniProtKB: PDZK1-interacting protein 1 |
-Macromolecule #3: N-[2-(dimethylamino)ethyl]-2-methyl-2-[4-[4-[[2-methyl-5-[(2S,3R,...
| Macromolecule | Name: N-[2-(dimethylamino)ethyl]-2-methyl-2-[4-[4-[[2-methyl-5-[(2S,3R,4R,5S,6R)-6-methylsulfanyl-3,4,5-tris(oxidanyl)oxan-2-yl]phenyl]methyl]phenyl]butanoylamino]propanamide type: ligand / ID: 3 / Number of copies: 1 / Formula: 1YI |
|---|---|
| Molecular weight | Theoretical: 601.797 Da |
| Chemical component information | 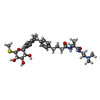 ChemComp-1YI: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE / Details: ab initio |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. v3.1.0) / Number images used: 12133 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





