[English] 日本語
 Yorodumi
Yorodumi- EMDB-32283: Structure of USP14-bound human 26S proteasome in substrate-inhibi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of USP14-bound human 26S proteasome in substrate-inhibited state SD4_USP14 | |||||||||
 Map data Map data | Complete map of the human proteasome holoenzyme in state SD4_USP14, with the RP/CP low-pass filtered to 4.6/3.0 angstrom and sharpened by a B-factor of -30/0 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Proteasome / AAA-ATPase / Deubiquitinase / USP14 / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of ERAD pathway / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / deubiquitinase activity / regulation of chemotaxis / protein K48-linked deubiquitination ...negative regulation of ERAD pathway / thyrotropin-releasing hormone receptor binding / nuclear proteasome complex / host-mediated perturbation of viral transcription / positive regulation of inclusion body assembly / Impaired BRCA2 translocation to the nucleus / Impaired BRCA2 binding to SEM1 (DSS1) / deubiquitinase activity / regulation of chemotaxis / protein K48-linked deubiquitination / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / proteasome accessory complex / integrator complex / purine ribonucleoside triphosphate binding / meiosis I / proteasome regulatory particle / cytosolic proteasome complex / positive regulation of proteasomal protein catabolic process / proteasome-activating activity / proteasome regulatory particle, lid subcomplex / proteasome regulatory particle, base subcomplex / hypothalamus gonadotrophin-releasing hormone neuron development / metal-dependent deubiquitinase activity / protein K63-linked deubiquitination / female meiosis I / negative regulation of programmed cell death / Regulation of ornithine decarboxylase (ODC) / positive regulation of protein monoubiquitination / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / fat pad development / proteasome core complex / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / mitochondrion transport along microtubule / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / endopeptidase inhibitor activity / Somitogenesis / Resolution of D-loop Structures through Holliday Junction Intermediates / K63-linked deubiquitinase activity / female gonad development / Impaired BRCA2 binding to RAD51 / seminiferous tubule development / proteasome binding / transcription factor binding / regulation of protein catabolic process / myofibril / male meiosis I / proteasome storage granule / Presynaptic phase of homologous DNA pairing and strand exchange / positive regulation of intrinsic apoptotic signaling pathway by p53 class mediator / general transcription initiation factor binding / blastocyst development / negative regulation of ubiquitin-dependent protein catabolic process / polyubiquitin modification-dependent protein binding / immune system process / protein deubiquitination / NF-kappaB binding / proteasome endopeptidase complex / endopeptidase activator activity / proteasome core complex, beta-subunit complex / proteasome assembly / threonine-type endopeptidase activity / mRNA export from nucleus / proteasome core complex, alpha-subunit complex / energy homeostasis / presynaptic cytosol / regulation of neuron apoptotic process / neuron projection morphogenesis / enzyme regulator activity / regulation of proteasomal protein catabolic process / Maturation of protein E / Maturation of protein E / inclusion body / ER Quality Control Compartment (ERQC) / Myoclonic epilepsy of Lafora / FLT3 signaling by CBL mutants / Prevention of phagosomal-lysosomal fusion / IRAK2 mediated activation of TAK1 complex / Alpha-protein kinase 1 signaling pathway / Glycogen synthesis / IRAK1 recruits IKK complex / IRAK1 recruits IKK complex upon TLR7/8 or 9 stimulation / Endosomal Sorting Complex Required For Transport (ESCRT) / Membrane binding and targetting of GAG proteins / Negative regulation of FLT3 / Regulation of TBK1, IKKε (IKBKE)-mediated activation of IRF3, IRF7 / PTK6 Regulates RTKs and Their Effectors AKT1 and DOK1 / Regulation of TBK1, IKKε-mediated activation of IRF3, IRF7 upon TLR3 ligation / Constitutive Signaling by NOTCH1 HD Domain Mutants / IRAK2 mediated activation of TAK1 complex upon TLR7/8 or 9 stimulation / NOTCH2 Activation and Transmission of Signal to the Nucleus / TICAM1,TRAF6-dependent induction of TAK1 complex / TICAM1-dependent activation of IRF3/IRF7 / APC/C:Cdc20 mediated degradation of Cyclin B / Downregulation of ERBB4 signaling / Regulation of FZD by ubiquitination / APC-Cdc20 mediated degradation of Nek2A Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Zhang S / Zou S / Yin D / Wu Z / Mao Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: USP14-regulated allostery of the human proteasome by time-resolved cryo-EM. Authors: Shuwen Zhang / Shitao Zou / Deyao Yin / Lihong Zhao / Daniel Finley / Zhaolong Wu / Youdong Mao /   Abstract: Proteasomal degradation of ubiquitylated proteins is tightly regulated at multiple levels. A primary regulatory checkpoint is the removal of ubiquitin chains from substrates by the deubiquitylating ...Proteasomal degradation of ubiquitylated proteins is tightly regulated at multiple levels. A primary regulatory checkpoint is the removal of ubiquitin chains from substrates by the deubiquitylating enzyme ubiquitin-specific protease 14 (USP14), which reversibly binds the proteasome and confers the ability to edit and reject substrates. How USP14 is activated and regulates proteasome function remain unknown. Here we present high-resolution cryo-electron microscopy structures of human USP14 in complex with the 26S proteasome in 13 distinct conformational states captured during degradation of polyubiquitylated proteins. Time-resolved cryo-electron microscopy analysis of the conformational continuum revealed two parallel pathways of proteasome state transitions induced by USP14, and captured transient conversion of substrate-engaged intermediates into substrate-inhibited intermediates. On the substrate-engaged pathway, ubiquitin-dependent activation of USP14 allosterically reprograms the conformational landscape of the AAA-ATPase motor and stimulates opening of the core particle gate, enabling observation of a near-complete cycle of asymmetric ATP hydrolysis around the ATPase ring during processive substrate unfolding. Dynamic USP14-ATPase interactions decouple the ATPase activity from RPN11-catalysed deubiquitylation and kinetically introduce three regulatory checkpoints on the proteasome, at the steps of ubiquitin recognition, substrate translocation initiation and ubiquitin chain recycling. These findings provide insights into the complete functional cycle of the USP14-regulated proteasome and establish mechanistic foundations for the discovery of USP14-targeted therapies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32283.map.gz emd_32283.map.gz | 892.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32283-v30.xml emd-32283-v30.xml emd-32283.xml emd-32283.xml | 70.7 KB 70.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32283.png emd_32283.png | 69.1 KB | ||
| Filedesc metadata |  emd-32283.cif.gz emd-32283.cif.gz | 15.1 KB | ||
| Others |  emd_32283_additional_1.map.gz emd_32283_additional_1.map.gz emd_32283_additional_2.map.gz emd_32283_additional_2.map.gz emd_32283_additional_3.map.gz emd_32283_additional_3.map.gz emd_32283_additional_4.map.gz emd_32283_additional_4.map.gz | 900.8 MB 870.3 MB 894.5 MB 870.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32283 http://ftp.pdbj.org/pub/emdb/structures/EMD-32283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32283 | HTTPS FTP |
-Validation report
| Summary document |  emd_32283_validation.pdf.gz emd_32283_validation.pdf.gz | 636.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32283_full_validation.pdf.gz emd_32283_full_validation.pdf.gz | 636.5 KB | Display | |
| Data in XML |  emd_32283_validation.xml.gz emd_32283_validation.xml.gz | 8.5 KB | Display | |
| Data in CIF |  emd_32283_validation.cif.gz emd_32283_validation.cif.gz | 9.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32283 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32283 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32283 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32283 | HTTPS FTP |
-Related structure data
| Related structure data |  7w3kMC  7w37C  7w38C  7w39C  7w3aC  7w3bC  7w3cC  7w3fC  7w3gC  7w3hC  7w3iC  7w3jC  7w3mC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32283.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32283.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complete map of the human proteasome holoenzyme in state SD4_USP14, with the RP/CP low-pass filtered to 4.6/3.0 angstrom and sharpened by a B-factor of -30/0 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.685 Å | ||||||||||||||||||||||||||||||||||||
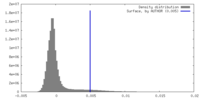
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Map of the human proteasome in state SD4 USP14...
| File | emd_32283_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of the human proteasome in state SD4_USP14 after CP-masked refinement, low-pass filtered to 3.0 angstrom with no sharpening B-factor applied | ||||||||||||
| Projections & Slices |
| ||||||||||||
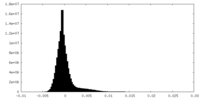
| Density Histograms |
-Additional map: Unfiltered, unsharpened raw map of the human proteasome...
| File | emd_32283_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered, unsharpened raw map of the human proteasome in state SD4_USP14 after CP-masked refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
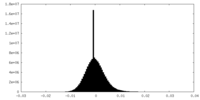
| Density Histograms |
-Additional map: Map of the human proteasome in state SD4 USP14...
| File | emd_32283_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of the human proteasome in state SD4_USP14 after RP-masked refinement, low-pass filtered to 4.6 angstrom and sharpened by a B-factor of -30 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unfiltered, unsharpened raw map of the human proteasome...
| File | emd_32283_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered, unsharpened raw map of the human proteasome in state SD4_USP14 after RP-masked refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : 26S proteasome
+Supramolecule #1: 26S proteasome
+Supramolecule #2: 26S proteasome
+Supramolecule #3: SD4_USP14
+Macromolecule #1: 26S protease regulatory subunit 7
+Macromolecule #2: 26S protease regulatory subunit 4
+Macromolecule #3: 26S proteasome regulatory subunit 10B
+Macromolecule #4: 26S protease regulatory subunit 6A
+Macromolecule #5: Proteasome subunit alpha type-6
+Macromolecule #6: Proteasome subunit alpha type-2
+Macromolecule #7: Proteasome subunit alpha type-4
+Macromolecule #8: Proteasome subunit alpha type-7
+Macromolecule #9: Proteasome subunit alpha type-5
+Macromolecule #10: Isoform Long of Proteasome subunit alpha type-1
+Macromolecule #11: Proteasome subunit alpha type-3
+Macromolecule #12: Proteasome subunit beta type-6
+Macromolecule #13: Proteasome subunit beta type-7
+Macromolecule #14: Proteasome subunit beta type-3
+Macromolecule #15: Proteasome subunit beta type-2
+Macromolecule #16: Proteasome subunit beta type-5
+Macromolecule #17: Proteasome subunit beta type-1
+Macromolecule #18: Proteasome subunit beta type-4
+Macromolecule #19: Ubiquitin carboxyl-terminal hydrolase 14
+Macromolecule #20: Ubiquitin
+Macromolecule #21: Isoform 2 of 26S proteasome regulatory subunit 8
+Macromolecule #22: 26S protease regulatory subunit 6B
+Macromolecule #23: 26S proteasome non-ATPase regulatory subunit 1
+Macromolecule #24: 26S proteasome non-ATPase regulatory subunit 12
+Macromolecule #25: 26S proteasome non-ATPase regulatory subunit 11
+Macromolecule #26: 26S proteasome non-ATPase regulatory subunit 6
+Macromolecule #27: 26S proteasome non-ATPase regulatory subunit 7
+Macromolecule #28: 26S proteasome non-ATPase regulatory subunit 13
+Macromolecule #29: 26S proteasome non-ATPase regulatory subunit 4
+Macromolecule #30: 26S proteasome non-ATPase regulatory subunit 14
+Macromolecule #31: 26S proteasome non-ATPase regulatory subunit 8
+Macromolecule #32: 26S proteasome non-ATPase regulatory subunit 2
+Macromolecule #33: 26S proteasome non-ATPase regulatory subunit 3
+Macromolecule #34: 26S proteasome complex subunit DSS1
+Macromolecule #35: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #36: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #37: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.4 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 74792 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)