[English] 日本語
 Yorodumi
Yorodumi- EMDB-32152: Cryo-EM structure of human very long-chain fatty acid ABC transpo... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human very long-chain fatty acid ABC transporter ABCD1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | very long-chain fatty / Peroxisome / ABC transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationABC transporters in lipid homeostasis / Linoleic acid (LA) metabolism / alpha-linolenic acid (ALA) metabolism / Beta-oxidation of very long chain fatty acids / Class I peroxisomal membrane protein import / ABC-type fatty-acyl-CoA transporter activity / peroxisomal membrane transport / very long-chain fatty-acyl-CoA catabolic process / very long-chain fatty acyl-CoA hydrolase activity / positive regulation of unsaturated fatty acid biosynthetic process ...ABC transporters in lipid homeostasis / Linoleic acid (LA) metabolism / alpha-linolenic acid (ALA) metabolism / Beta-oxidation of very long chain fatty acids / Class I peroxisomal membrane protein import / ABC-type fatty-acyl-CoA transporter activity / peroxisomal membrane transport / very long-chain fatty-acyl-CoA catabolic process / very long-chain fatty acyl-CoA hydrolase activity / positive regulation of unsaturated fatty acid biosynthetic process / Linoleic acid (LA) metabolism / Defective ABCD1 causes ALD / long-chain fatty acid catabolic process / long-chain fatty acid import into peroxisome / very long-chain fatty acid catabolic process / alpha-linolenic acid (ALA) metabolism / regulation of fatty acid beta-oxidation / Beta-oxidation of very long chain fatty acids / fatty acid derivative biosynthetic process / alpha-linolenic acid metabolic process / sterol homeostasis / very long-chain fatty acid metabolic process / Class I peroxisomal membrane protein import / unsaturated fatty acid biosynthetic process / peroxisome organization / fatty acyl-CoA hydrolase activity / regulation of mitochondrial depolarization / ABC transporters in lipid homeostasis / myelin maintenance / regulation of oxidative phosphorylation / regulation of cellular response to oxidative stress / positive regulation of fatty acid beta-oxidation / linoleic acid metabolic process / Hydrolases; Acting on ester bonds; Thioester hydrolases / fatty acid elongation / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / peroxisomal membrane / long-chain fatty acid biosynthetic process / long-chain fatty acid transmembrane transporter activity / fatty acid beta-oxidation / ATPase-coupled transmembrane transporter activity / fatty acid homeostasis / ABC-type transporter activity / negative regulation of reactive oxygen species biosynthetic process / neuron projection maintenance / negative regulation of cytokine production involved in inflammatory response / ADP binding / mitochondrial membrane / peroxisome / lysosomal membrane / endoplasmic reticulum membrane / perinuclear region of cytoplasm / enzyme binding / protein homodimerization activity / ATP hydrolysis activity / ATP binding / identical protein binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.53 Å | |||||||||
 Authors Authors | Chen ZP / Xu D | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural basis of substrate recognition and translocation by human very long-chain fatty acid transporter ABCD1. Authors: Zhi-Peng Chen / Da Xu / Liang Wang / Yao-Xu Mao / Yang Li / Meng-Ting Cheng / Cong-Zhao Zhou / Wen-Tao Hou / Yuxing Chen /  Abstract: Human ABC transporter ABCD1 transports very long-chain fatty acids from cytosol to peroxisome for β-oxidation, dysfunction of which usually causes the X-linked adrenoleukodystrophy (X-ALD). Here, we ...Human ABC transporter ABCD1 transports very long-chain fatty acids from cytosol to peroxisome for β-oxidation, dysfunction of which usually causes the X-linked adrenoleukodystrophy (X-ALD). Here, we report three cryogenic electron microscopy structures of ABCD1: the apo-form, substrate- and ATP-bound forms. Distinct from what was seen in the previously reported ABC transporters, the two symmetric molecules of behenoyl coenzyme A (C22:0-CoA) cooperatively bind to the transmembrane domains (TMDs). For each C22:0-CoA, the hydrophilic 3'-phospho-ADP moiety of CoA portion inserts into one TMD, with the succeeding pantothenate and cysteamine moiety crossing the inter-domain cavity, whereas the hydrophobic fatty acyl chain extends to the opposite TMD. Structural analysis combined with biochemical assays illustrates snapshots of ABCD1-mediated substrate transport cycle. It advances our understanding on the selective oxidation of fatty acids and molecular pathology of X-ALD. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32152.map.gz emd_32152.map.gz | 37.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32152-v30.xml emd-32152-v30.xml emd-32152.xml emd-32152.xml | 11.8 KB 11.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32152.png emd_32152.png | 146.9 KB | ||
| Filedesc metadata |  emd-32152.cif.gz emd-32152.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32152 http://ftp.pdbj.org/pub/emdb/structures/EMD-32152 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32152 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32152 | HTTPS FTP |
-Validation report
| Summary document |  emd_32152_validation.pdf.gz emd_32152_validation.pdf.gz | 600.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32152_full_validation.pdf.gz emd_32152_full_validation.pdf.gz | 600 KB | Display | |
| Data in XML |  emd_32152_validation.xml.gz emd_32152_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_32152_validation.cif.gz emd_32152_validation.cif.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32152 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32152 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32152 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32152 | HTTPS FTP |
-Related structure data
| Related structure data |  7vwcMC  7vx8C  7vzbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32152.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32152.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : human peroxisomal ABCD1
| Entire | Name: human peroxisomal ABCD1 |
|---|---|
| Components |
|
-Supramolecule #1: human peroxisomal ABCD1
| Supramolecule | Name: human peroxisomal ABCD1 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 175.6 kDa/nm |
-Macromolecule #1: Peroxisomal Membrane Protein related,ATP-binding cassette sub-fam...
| Macromolecule | Name: Peroxisomal Membrane Protein related,ATP-binding cassette sub-family D member 1 type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 87.931 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MASWSHPQFE KGGGARGGSG GGSWSHPQFE KGFDYKDDDD KGTSVQSKFL ETGIDPEKRK KALLVGAAGV AAVAVAHWVS LRKSQKSHH KMEKEDSVAS DGTPKKNKKA GMNRVFLQRL LWLLRLLFPR VLCRETGLLA LHSAALVSRT FLSVYVARLD G RLARCIVR ...String: MASWSHPQFE KGGGARGGSG GGSWSHPQFE KGFDYKDDDD KGTSVQSKFL ETGIDPEKRK KALLVGAAGV AAVAVAHWVS LRKSQKSHH KMEKEDSVAS DGTPKKNKKA GMNRVFLQRL LWLLRLLFPR VLCRETGLLA LHSAALVSRT FLSVYVARLD G RLARCIVR KDPRAFGWQL LQWLLIALPA TFVNSAIRYL EGQLALSFRS RLVAHAYRLY FSQQTYYRVS NMDGRLRNPD QS LTEDVVA FAASVAHLYS NLTKPLLDVA VTSYTLLRAA RSRGAGTAWP SAIAGLVVFL TANVLRAFSP KFGELVAEEA RRK GELRYM HSRVVANSEE IAFYGGHEVE LALLQRSYQD LASQINLILL ERLWYVMLEQ FLMKYVWSAS GLLMVAVPII TATG YSESD AEAVKKAALE KKEEELVSER TEAFTIARNL LTAAADAIER IMSSYKEVTE LAGYTARVHE MFQVFEDVQR CHFKR PREL EDAQAGSGTI GRSGVRVEGP LKIRGQVVDV EQGIICENIP IVTPSGEVVV ASLNIRVEEG MHLLITGPNG CGKSSL FRI LGGLWPTYGG VLYKPPPQRM FYIPQRPYMS VGSLRDQVIY PDSVEDMQRK GYSEQDLEAI LDVVHLHHIL QREGGWE AM CDWKDVLSGG EKQRIGMARM FYHRPKYALL DECTSAVSID VEGKIFQAAK DAGIALLSIT HRPSLWKYHT HLLQFDGE G GWKFEKLDSA ARLSLTEEKQ RLEQQLAGIP KMQRRLQELC QILGEAVAPA HVPAPSPQGP GGLQGAST UniProtKB: ABC transporter domain-containing protein, ATP-binding cassette sub-family D member 1 |
-Macromolecule #2: [(2R)-3-[2-azanylethoxy(oxidanyl)phosphoryl]oxy-2-oxidanyl-propyl...
| Macromolecule | Name: [(2R)-3-[2-azanylethoxy(oxidanyl)phosphoryl]oxy-2-oxidanyl-propyl] octadecanoate type: ligand / ID: 2 / Number of copies: 2 / Formula: 82T |
|---|---|
| Molecular weight | Theoretical: 481.603 Da |
| Chemical component information | 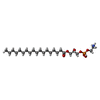 ChemComp-82T: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 8 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7vwc: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















