[English] 日本語
 Yorodumi
Yorodumi- EMDB-30591: Cryo-EM structure of the nucleosome containing Giardia histones -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30591 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the nucleosome containing Giardia histones | |||||||||
 Map data Map data | Nucleosome containing G. lamblia histones | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nucleosome / chromatin / histone / Giardia lamblia / NUCLEAR PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationstructural constituent of chromatin / nucleosome / protein heterodimerization activity / DNA binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Giardia intestinalis (eukaryote) / synthetic construct (others) Giardia intestinalis (eukaryote) / synthetic construct (others) | |||||||||
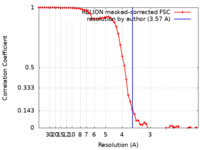
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||
 Authors Authors | Sato S / Takizawa Y | |||||||||
| Funding support |  Japan, 1 items Japan, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Cryo-EM structure of the nucleosome core particle containing Giardia lamblia histones. Authors: Shoko Sato / Yoshimasa Takizawa / Fumika Hoshikawa / Mariko Dacher / Hiroki Tanaka / Hiroaki Tachiwana / Tomoya Kujirai / Yukari Iikura / Cheng-Han Ho / Naruhiko Adachi / Indu Patwal / ...Authors: Shoko Sato / Yoshimasa Takizawa / Fumika Hoshikawa / Mariko Dacher / Hiroki Tanaka / Hiroaki Tachiwana / Tomoya Kujirai / Yukari Iikura / Cheng-Han Ho / Naruhiko Adachi / Indu Patwal / Andrew Flaus / Hitoshi Kurumizaka /   Abstract: Giardia lamblia is a pathogenic unicellular eukaryotic parasite that causes giardiasis. Its genome encodes the canonical histones H2A, H2B, H3, and H4, which share low amino acid sequence identity ...Giardia lamblia is a pathogenic unicellular eukaryotic parasite that causes giardiasis. Its genome encodes the canonical histones H2A, H2B, H3, and H4, which share low amino acid sequence identity with their human orthologues. We determined the structure of the G. lamblia nucleosome core particle (NCP) at 3.6 Å resolution by cryo-electron microscopy. G. lamblia histones form a characteristic NCP, in which the visible 125 base-pair region of the DNA is wrapped in a left-handed supercoil. The acidic patch on the G. lamblia octamer is deeper, due to an insertion extending the H2B α1 helix and L1 loop, and thus cannot bind the LANA acidic patch binding peptide. The DNA and histone regions near the DNA entry-exit sites could not be assigned, suggesting that these regions are asymmetrically flexible in the G. lamblia NCP. Characterization by thermal unfolding in solution revealed that both the H2A-H2B and DNA association with the G. lamblia H3-H4 were weaker than those for human H3-H4. These results demonstrate the uniformity of the histone octamer as the organizing platform for eukaryotic chromatin, but also illustrate the unrecognized capability for large scale sequence variations that enable the adaptability of histone octamer surfaces and confer internal stability. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30591.map.gz emd_30591.map.gz | 1.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30591-v30.xml emd-30591-v30.xml emd-30591.xml emd-30591.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30591_fsc.xml emd_30591_fsc.xml | 5.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_30591.png emd_30591.png | 181.9 KB | ||
| Filedesc metadata |  emd-30591.cif.gz emd-30591.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30591 http://ftp.pdbj.org/pub/emdb/structures/EMD-30591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30591 | HTTPS FTP |
-Related structure data
| Related structure data |  7d69MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30591.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30591.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Nucleosome containing G. lamblia histones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Nucleosome
| Entire | Name: Nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: Nucleosome
| Supramolecule | Name: Nucleosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Giardia intestinalis (eukaryote) Giardia intestinalis (eukaryote) |
-Macromolecule #1: Histone H3
| Macromolecule | Name: Histone H3 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Giardia intestinalis (eukaryote) Giardia intestinalis (eukaryote) |
| Molecular weight | Theoretical: 16.639574 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMARTKHT ARKTTSATKA PRKTIARKAA RKTASSTSGI KKTGRKKQGM VAVKEIKKYQ KSTDLLIRKL PFSKLVRDIV TSGLSKSDI RFQGAAVEAL QESAENYIIS LFVDTQLCAE HAKRVTIMKP DMELATRIGK RIEPEYRKGK UniProtKB: Histone H3 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Giardia intestinalis (eukaryote) Giardia intestinalis (eukaryote) |
| Molecular weight | Theoretical: 11.407195 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMSGKGKG KGYGKSKRHS KEKDTLGGIT KPAIRRLARR GGVKRISSTI YQQTREVLKA FLEVVLRDSL TYTEHGQRKT VTSQDVVYA LKRQGRTLYG FGI UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2B
| Macromolecule | Name: Histone H2B / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Giardia intestinalis (eukaryote) Giardia intestinalis (eukaryote) |
| Molecular weight | Theoretical: 14.903089 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMSKVETK RLMKKTEAGD KGDAKRKHKR HETYATYIYK VLRSENIRSE ADTDLGISNK GMEVMNSLVN DLFERIASEA SNLAKISKR NTIGKKDIES AAKLVIPGEI GRLIRDEADK ALSKFTSSKE STKK UniProtKB: Histone H2B |
-Macromolecule #4: Histone H2A
| Macromolecule | Name: Histone H2A / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Giardia intestinalis (eukaryote) Giardia intestinalis (eukaryote) |
| Molecular weight | Theoretical: 14.188249 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSHMSTKPVK DNSKMKSRSA RAGISFPIGR IHRHLREGRY AERISSDAPV YLAAVLENVV AEVFREACNH RDKKSQKRIV PNHILTALR KDKELATIFA NVTIREGGVA RSAKEGREGK GSHRSQDL UniProtKB: Histone H2A |
-Macromolecule #5: 601L DNA (145-MER)
| Macromolecule | Name: 601L DNA (145-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 44.761523 KDa |
| Sequence | String: (DA)(DT)(DC)(DA)(DC)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC) ...String: (DA)(DT)(DC)(DA)(DC)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC)(DT)(DA) (DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT)(DA) (DA)(DA) (DC)(DG)(DC)(DA)(DC)(DG)(DT) (DA)(DC)(DG)(DG)(DA)(DA)(DT)(DC)(DC)(DG) (DT)(DA)(DC) (DG)(DT)(DG)(DC)(DG)(DT) (DT)(DT)(DA)(DA)(DG)(DC)(DG)(DG)(DT)(DG) (DC)(DT)(DA)(DG) (DA)(DG)(DC)(DT)(DG) (DT)(DC)(DT)(DA)(DC)(DG)(DA)(DC)(DC)(DA) (DA)(DT)(DT)(DG)(DA) (DG)(DC)(DG)(DG) (DC)(DC)(DT)(DC)(DG)(DG)(DC)(DA)(DC)(DC) (DG)(DG)(DG)(DA)(DT)(DT) (DG)(DT)(DG) (DA)(DT) |
-Macromolecule #6: 601L DNA (145-MER)
| Macromolecule | Name: 601L DNA (145-MER) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 44.752508 KDa |
| Sequence | String: (DA)(DT)(DC)(DA)(DC)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC) ...String: (DA)(DT)(DC)(DA)(DC)(DA)(DA)(DT)(DC)(DC) (DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA)(DG) (DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA)(DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA)(DG) (DA) (DC)(DA)(DG)(DC)(DT)(DC)(DT)(DA) (DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT)(DA) (DA)(DA) (DC)(DG)(DC)(DA)(DC)(DG)(DT) (DA)(DC)(DG)(DG)(DA)(DT)(DT)(DC)(DC)(DG) (DT)(DA)(DC) (DG)(DT)(DG)(DC)(DG)(DT) (DT)(DT)(DA)(DA)(DG)(DC)(DG)(DG)(DT)(DG) (DC)(DT)(DA)(DG) (DA)(DG)(DC)(DT)(DG) (DT)(DC)(DT)(DA)(DC)(DG)(DA)(DC)(DC)(DA) (DA)(DT)(DT)(DG)(DA) (DG)(DC)(DG)(DG) (DC)(DC)(DT)(DC)(DG)(DG)(DC)(DA)(DC)(DC) (DG)(DG)(DG)(DA)(DT)(DT) (DG)(DT)(DG) (DA)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7d69: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)