+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2983 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo EM structure of suilysin pore | |||||||||
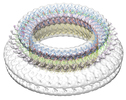
 Map data Map data | reconstruction of suilysin pore on liposome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cholesterol dependent cytolysin / hemolysin / toxin | |||||||||
| Function / homology | :  Function and homology information Function and homology information | |||||||||
| Biological species |  Streptococcus suis (bacteria) Streptococcus suis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 15.0 Å | |||||||||
 Authors Authors | Dudkina NV / Leung C / Lukoyanova N / Hodel AW / Farabella I / Pandurangan AP / Jahan N / Pires Damaso M / Osmanovic D / Reboul CF ...Dudkina NV / Leung C / Lukoyanova N / Hodel AW / Farabella I / Pandurangan AP / Jahan N / Pires Damaso M / Osmanovic D / Reboul CF / Dunstone MA / Andrew PW / Lonnen R / Topf M / Saibil HR / Hoogenboom BW | |||||||||
 Citation Citation |  Journal: Elife / Year: 2014 Journal: Elife / Year: 2014Title: Stepwise visualization of membrane pore formation by suilysin, a bacterial cholesterol-dependent cytolysin. Authors: Carl Leung / Natalya V Dudkina / Natalya Lukoyanova / Adrian W Hodel / Irene Farabella / Arun P Pandurangan / Nasrin Jahan / Mafalda Pires Damaso / Dino Osmanović / Cyril F Reboul / ...Authors: Carl Leung / Natalya V Dudkina / Natalya Lukoyanova / Adrian W Hodel / Irene Farabella / Arun P Pandurangan / Nasrin Jahan / Mafalda Pires Damaso / Dino Osmanović / Cyril F Reboul / Michelle A Dunstone / Peter W Andrew / Rana Lonnen / Maya Topf / Helen R Saibil / Bart W Hoogenboom /   Abstract: Membrane attack complex/perforin/cholesterol-dependent cytolysin (MACPF/CDC) proteins constitute a major superfamily of pore-forming proteins that act as bacterial virulence factors and effectors in ...Membrane attack complex/perforin/cholesterol-dependent cytolysin (MACPF/CDC) proteins constitute a major superfamily of pore-forming proteins that act as bacterial virulence factors and effectors in immune defence. Upon binding to the membrane, they convert from the soluble monomeric form to oligomeric, membrane-inserted pores. Using real-time atomic force microscopy (AFM), electron microscopy (EM), and atomic structure fitting, we have mapped the structure and assembly pathways of a bacterial CDC in unprecedented detail and accuracy, focussing on suilysin from Streptococcus suis. We show that suilysin assembly is a noncooperative process that is terminated before the protein inserts into the membrane. The resulting ring-shaped pores and kinetically trapped arc-shaped assemblies are all seen to perforate the membrane, as also visible by the ejection of its lipids. Membrane insertion requires a concerted conformational change of the monomeric subunits, with a marked expansion in pore diameter due to large changes in subunit structure and packing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2983.map.gz emd_2983.map.gz | 17.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2983-v30.xml emd-2983-v30.xml emd-2983.xml emd-2983.xml | 11.3 KB 11.3 KB | Display Display |  EMDB header EMDB header |
| Images |  Slypore.jpg Slypore.jpg | 207.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2983 http://ftp.pdbj.org/pub/emdb/structures/EMD-2983 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2983 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2983 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2983.map.gz / Format: CCP4 / Size: 238.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2983.map.gz / Format: CCP4 / Size: 238.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | reconstruction of suilysin pore on liposome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Suilysin
| Entire | Name: Suilysin |
|---|---|
| Components |
|
-Supramolecule #1000: Suilysin
| Supramolecule | Name: Suilysin / type: sample / ID: 1000 Details: The protein was incubated with lipid vesicles (PC:Cholesterol, molar ratio 45:55) for 10 minutes at 37oC Oligomeric state: 37 / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 2.072 MDa / Theoretical: 2.072 MDa / Method: Calculated from the molecular weight of the monomer |
-Macromolecule #1: suilysin
| Macromolecule | Name: suilysin / type: protein_or_peptide / ID: 1 / Name.synonym: SLY / Number of copies: 1 / Oligomeric state: 37 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Streptococcus suis (bacteria) / Cell: bacterium Streptococcus suis (bacteria) / Cell: bacterium |
| Molecular weight | Experimental: 56 KDa / Theoretical: 56 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: UNIPROTKB: C6GNG6 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 50 mM HEPES, 100 mM NaCl |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 91 K / Instrument: FEI VITROBOT MARK III Method: Liposomes with the protein were applied to glow-discharged lacey carbon coated copper grids and blotted for 5 s. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 80 K / Max: 90 K / Average: 85 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 150,000x magnification |
| Date | Dec 1, 2013 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Sampling interval: 15 µm / Number real images: 7128 / Average electron dose: 25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 75000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.3 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder model: GATAN HELIUM |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | 37-fold symmetrised reconstruction. Image regions containing the pores with small surrounding areas of membrane were selected manually using Boxer (EMAN 1.9) software. |
|---|---|
| CTF correction | Details: Phase flipping for each particle |
| Final reconstruction | Applied symmetry - Point group: C37 (37 fold cyclic) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 15.0 Å / Resolution method: OTHER / Software - Name: SPIDER, IMAGIC, EMAN / Details: Final maps were calculated by BP RP in SPIDER / Number images used: 600 |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Rigid body fitting of domain models created as described in Leung et al (2014) eLife 3:e04247 |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross-correlation coefficient |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















