+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Semi-synthetic CoA-alpha-Synuclein Constructs Trap N-terminal Acetyltransferase NatB for Binding Mechanism Studies | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | N-terminal acetyltransferase / TRANSFERASE | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報N-terminal peptidyl-glutamine acetylation / N-terminal methionine Nalpha-acetyltransferase NatB / N-terminal peptidyl-aspartic acid acetylation / N-terminal peptidyl-glutamic acid acetylation / NatB complex / N-terminal protein amino acid acetylation / protein N-terminal-methionine acetyltransferase activity / protein-N-terminal amino-acid acetyltransferase activity / acetyltransferase activator activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone ...N-terminal peptidyl-glutamine acetylation / N-terminal methionine Nalpha-acetyltransferase NatB / N-terminal peptidyl-aspartic acid acetylation / N-terminal peptidyl-glutamic acid acetylation / NatB complex / N-terminal protein amino acid acetylation / protein N-terminal-methionine acetyltransferase activity / protein-N-terminal amino-acid acetyltransferase activity / acetyltransferase activator activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / regulation of synaptic vesicle recycling / negative regulation of chaperone-mediated autophagy / mitochondrial membrane organization / regulation of reactive oxygen species biosynthetic process / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / SNARE complex assembly / positive regulation of neurotransmitter secretion / regulation of locomotion / negative regulation of dopamine metabolic process / positive regulation of inositol phosphate biosynthetic process / regulation of macrophage activation / negative regulation of microtubule polymerization / regulation of norepinephrine uptake / synaptic vesicle transport / transporter regulator activity / synaptic vesicle priming / dopamine uptake involved in synaptic transmission / protein kinase inhibitor activity / mitochondrial ATP synthesis coupled electron transport / regulation of dopamine secretion / dynein complex binding / negative regulation of thrombin-activated receptor signaling pathway / positive regulation of receptor recycling / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of endocytosis / positive regulation of exocytosis / synaptic vesicle exocytosis / kinesin binding / synaptic vesicle endocytosis / enzyme inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of serotonin uptake / response to type II interferon / regulation of presynapse assembly / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / supramolecular fiber organization / phospholipid metabolic process / cellular response to fibroblast growth factor stimulus / inclusion body / axon terminus / Hsp70 protein binding / cellular response to epinephrine stimulus / cytoskeleton organization / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / adult locomotory behavior / SNARE binding / excitatory postsynaptic potential / regulation of actin cytoskeleton organization / protein tetramerization / phosphoprotein binding / microglial cell activation / ferrous iron binding / fatty acid metabolic process / regulation of long-term neuronal synaptic plasticity / synapse organization / protein destabilization / PKR-mediated signaling / phospholipid binding / receptor internalization / tau protein binding / long-term synaptic potentiation / terminal bouton / positive regulation of inflammatory response / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
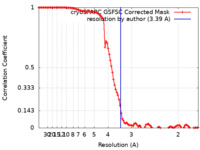
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.39 Å | |||||||||
 データ登録者 データ登録者 | Gardner SM / Marmorstein R | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 | ジャーナル: bioRxiv / 年: 2023 タイトル: Semi-synthetic CoA-α-Synuclein Constructs Trap N-terminal Acetyltransferase NatB for Binding Mechanism Studies. 著者: Buyan Pan / Sarah Gardner / Kollin Schultz / Ryann M Perez / Sunbin Deng / Marie Shimogawa / Kohei Sato / Elizabeth Rhoades / Ronen Marmorstein / E James Petersson 要旨: N-terminal acetylation is a chemical modification carried out by N-terminal acetyltransferases (NATs). A major member of this enzyme family, NatB, acts on much of the human proteome, including α- ...N-terminal acetylation is a chemical modification carried out by N-terminal acetyltransferases (NATs). A major member of this enzyme family, NatB, acts on much of the human proteome, including α-synuclein (αS), a synaptic protein that mediates vesicle trafficking. NatB acetylation of αS modulates its lipid vesicle binding properties and amyloid fibril formation, which underlies its role in the pathogenesis of Parkinson's disease. Although the molecular details of the interaction between human NatB (hNatB) and the N-terminus of αS have been resolved, whether the remainder of the protein plays a role in interacting with the enzyme is unknown. Here we execute the first synthesis, by native chemical ligation, of a bisubstrate inhibitor of NatB consisting of coenzyme A and full-length human αS, additionally incorporating two fluorescent probes for studies of conformational dynamics. We use cryo-electron microscopy (cryo-EM) to characterize the structural features of the hNatB/inhibitor complex and show that, beyond the first few residues, αS remains disordered when in complex with hNatB. We further probe changes in the αS conformation by single molecule Förster resonance energy transfer (smFRET) to reveal that the C-terminus expands when bound to hNatB. Computational models based on the cryo-EM and smFRET data help to explain the conformational changes and their implications for hNatB substrate recognition and specific inhibition of the interaction with αS. Beyond the study of αS and NatB, these experiments illustrate valuable strategies for the study of challenging structural biology targets through a combination of protein semi-synthesis, cryo-EM, smFRET, and computational modeling. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_29657.map.gz emd_29657.map.gz | 51.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-29657-v30.xml emd-29657-v30.xml emd-29657.xml emd-29657.xml | 18.6 KB 18.6 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_29657_fsc.xml emd_29657_fsc.xml | 9.9 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_29657.png emd_29657.png | 48.5 KB | ||
| Filedesc metadata |  emd-29657.cif.gz emd-29657.cif.gz | 6.3 KB | ||
| その他 |  emd_29657_half_map_1.map.gz emd_29657_half_map_1.map.gz emd_29657_half_map_2.map.gz emd_29657_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29657 http://ftp.pdbj.org/pub/emdb/structures/EMD-29657 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29657 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29657 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_29657_validation.pdf.gz emd_29657_validation.pdf.gz | 900.8 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_29657_full_validation.pdf.gz emd_29657_full_validation.pdf.gz | 900.4 KB | 表示 | |
| XML形式データ |  emd_29657_validation.xml.gz emd_29657_validation.xml.gz | 18.3 KB | 表示 | |
| CIF形式データ |  emd_29657_validation.cif.gz emd_29657_validation.cif.gz | 23.6 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29657 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29657 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29657 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29657 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8g0lMC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_29657.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_29657.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #1
| ファイル | emd_29657_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_29657_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Ternary complex of NAA20, NAA25 and CoA-alpha-Synuclein
| 全体 | 名称: Ternary complex of NAA20, NAA25 and CoA-alpha-Synuclein |
|---|---|
| 要素 |
|
-超分子 #1: Ternary complex of NAA20, NAA25 and CoA-alpha-Synuclein
| 超分子 | 名称: Ternary complex of NAA20, NAA25 and CoA-alpha-Synuclein タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#3 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: N-alpha-acetyltransferase 20
| 分子 | 名称: N-alpha-acetyltransferase 20 / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO EC番号: N-terminal methionine Nalpha-acetyltransferase NatB |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 20.390133 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MTTLRAFTCD DLFRFNNINL DPLTETYGIP FYLQYLAHWP EYFIVAEAPG GELMGYIMGK AEGSVAREEW HGHVTALSVA PEFRRLGLA AKLMELLEEI SERKGGFFVD LFVRVSNQVA VNMYKQLGYS VYRTVIEYYS ASNGEPDEDA YDMRKALSRD T EKKSIIPL PHPVRPEDIE UniProtKB: N-alpha-acetyltransferase 20 |
-分子 #2: N-alpha-acetyltransferase 25, NatB auxiliary subunit
| 分子 | 名称: N-alpha-acetyltransferase 25, NatB auxiliary subunit タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 112.444258 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MATRGHVQDP NDRRLRPIYD YLDNGNNKMA IQQADKLLKK HKDLHCAKVL KAIGLQRTGK QEEAFTLAQE VAALEPTDDN SLQALTILY REMHRPELVT KLYEAAVKKV PNSEEYHSHL FMAYARVGEY KKMQQAGMAL YKIVPKNPYY FWSVMSLIMQ S ISAQDENL ...文字列: MATRGHVQDP NDRRLRPIYD YLDNGNNKMA IQQADKLLKK HKDLHCAKVL KAIGLQRTGK QEEAFTLAQE VAALEPTDDN SLQALTILY REMHRPELVT KLYEAAVKKV PNSEEYHSHL FMAYARVGEY KKMQQAGMAL YKIVPKNPYY FWSVMSLIMQ S ISAQDENL SKTMFLPLAE RMVEKMVKED KIEAEAEVEL YYMILERLGK YQEALDVIRG KLGEKLTSEI QSRENKCMAM YK KLSRWPE CNALSRRLLL KNSDDWQFYL TYFDSVFRLI EEAWSPPAEG EHSLEGEVHY SAEKAVKFIE DRITEESKSS RHL RGPHLA KLELIRRLRS QGCNDEYKLG DPEELMFQYF KKFGDKPCCF TDLKVFVDLL PATQCTKFIN QLLGVVPLST PTED KLALP ADIRALQQHL CVVQLTRLLG LYHTMDKNQK LSVVRELMLR YQHGLEFGKT CLKTELQFSD YYCLLAVHAL IDVWR ETGD ETTVWQALTL LEEGLTHSPS NAQFKLLLVR IYCMLGAFEP VVDLYSSLDA KHIQHDTIGY LLTRYAESLG QYAAAS QSC NFALRFFHSN QKDTSEYIIQ AYKYGAFEKI PEFIAFRNRL NNSLHFAQVR TERMLLDLLL EANISTSLAE SIKSMNL RP EEDDIPWEDL RDNRDLNVFF SWDPKDRDVS EEHKKLSLEE ETLWLRIRSL TLRLISGLPS LNHPVEPKNS EKTAENGV S SRIDILRLLL QQLEATLETG KRFIEKDIQY PFLGPVPTRM GGFFNSGCSQ CQISSFYLVN DIYELDTSGL EDTMEIQER IENSFKSLLD QLKDVFSKCK GDLLEVKDGN LKTHPTLLEN LVFFVETISV ILWVSSYCES VLRPYKLNLQ KKKKKKKETS IIMPPVFTS FQDYVTGLQT LISNVVDHIK GLETHLIALK LEELILEDTS LSPEERKFSK TVQGKVQSSY LHSLLEMGEL L KKRLETTK KLKI UniProtKB: N-alpha-acetyltransferase 25, NatB auxiliary subunit |
-分子 #3: Alpha-synuclein
| 分子 | 名称: Alpha-synuclein / タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 641.799 Da |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MDVFM UniProtKB: Alpha-synuclein |
-分子 #4: CARBOXYMETHYL COENZYME *A
| 分子 | 名称: CARBOXYMETHYL COENZYME *A / タイプ: ligand / ID: 4 / コピー数: 1 / 式: CMC |
|---|---|
| 分子量 | 理論値: 825.57 Da |
| Chemical component information |  ChemComp-CMC: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 4 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 撮影したグリッド数: 1 / 実像数: 5470 / 平均電子線量: 43.9 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.0 µm / 最小 デフォーカス(公称値): 1.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)