+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MicroED structure of A2A from plasma milled lamellae | |||||||||
 Map data Map data | Structure of the human A2A adenosine receptor from plasma milled lamellae | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | A2A Adenosine Receptor / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of norepinephrine secretion / positive regulation of acetylcholine secretion, neurotransmission / negative regulation of alpha-beta T cell activation / positive regulation of circadian sleep/wake cycle, sleep / Adenosine P1 receptors / G protein-coupled adenosine receptor activity / response to purine-containing compound / G protein-coupled adenosine receptor signaling pathway / NGF-independant TRKA activation / Surfactant metabolism ...regulation of norepinephrine secretion / positive regulation of acetylcholine secretion, neurotransmission / negative regulation of alpha-beta T cell activation / positive regulation of circadian sleep/wake cycle, sleep / Adenosine P1 receptors / G protein-coupled adenosine receptor activity / response to purine-containing compound / G protein-coupled adenosine receptor signaling pathway / NGF-independant TRKA activation / Surfactant metabolism / sensory perception / synaptic transmission, dopaminergic / type 5 metabotropic glutamate receptor binding / negative regulation of vascular permeability / synaptic transmission, cholinergic / intermediate filament / presynaptic active zone / positive regulation of glutamate secretion / positive regulation of urine volume / response to caffeine / blood circulation / eating behavior / inhibitory postsynaptic potential / alpha-actinin binding / regulation of calcium ion transport / asymmetric synapse / axolemma / membrane depolarization / prepulse inhibition / cellular defense response / phagocytosis / positive regulation of synaptic transmission, glutamatergic / neuron projection morphogenesis / astrocyte activation / presynaptic modulation of chemical synaptic transmission / central nervous system development / response to amphetamine / positive regulation of long-term synaptic potentiation / positive regulation of synaptic transmission, GABAergic / positive regulation of protein secretion / regulation of mitochondrial membrane potential / positive regulation of apoptotic signaling pathway / excitatory postsynaptic potential / synaptic transmission, glutamatergic / apoptotic signaling pathway / locomotory behavior / electron transport chain / negative regulation of inflammatory response / vasodilation / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / blood coagulation / cell-cell signaling / presynaptic membrane / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / negative regulation of neuron apoptotic process / postsynaptic membrane / calmodulin binding / electron transfer activity / periplasmic space / positive regulation of ERK1 and ERK2 cascade / iron ion binding / response to xenobiotic stimulus / inflammatory response / negative regulation of cell population proliferation / neuronal cell body / apoptotic process / heme binding / dendrite / regulation of DNA-templated transcription / lipid binding / protein-containing complex binding / glutamatergic synapse / enzyme binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | electron crystallography / cryo EM / Resolution: 2.0 Å | |||||||||
 Authors Authors | Martynowycz MW / Shiriaeva A / Clabbers MTB / Nicolas WJ / Weaver SJ / Hattne J / Gonen T | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A robust approach for MicroED sample preparation of lipidic cubic phase embedded membrane protein crystals. Authors: Michael W Martynowycz / Anna Shiriaeva / Max T B Clabbers / William J Nicolas / Sara J Weaver / Johan Hattne / Tamir Gonen /  Abstract: Crystallizing G protein-coupled receptors (GPCRs) in lipidic cubic phase (LCP) often yields crystals suited for the cryogenic electron microscopy (cryoEM) method microcrystal electron diffraction ...Crystallizing G protein-coupled receptors (GPCRs) in lipidic cubic phase (LCP) often yields crystals suited for the cryogenic electron microscopy (cryoEM) method microcrystal electron diffraction (MicroED). However, sample preparation is challenging. Embedded crystals cannot be targeted topologically. Here, we use an integrated fluorescence light microscope (iFLM) inside of a focused ion beam and scanning electron microscope (FIB-SEM) to identify fluorescently labeled GPCR crystals. Crystals are targeted using the iFLM and LCP is milled using a plasma focused ion beam (pFIB). The optimal ion source for preparing biological lamellae is identified using standard crystals of proteinase K. Lamellae prepared using either argon or xenon produced the highest quality data and structures. MicroED data are collected from the milled lamellae and the structures are determined. This study outlines a robust approach to identify and mill membrane protein crystals for MicroED and demonstrates plasma ion-beam milling is a powerful tool for preparing biological lamellae. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29586.map.gz emd_29586.map.gz | 13.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29586-v30.xml emd-29586-v30.xml emd-29586.xml emd-29586.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29586.png emd_29586.png | 62.6 KB | ||
| Filedesc metadata |  emd-29586.cif.gz emd-29586.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29586 http://ftp.pdbj.org/pub/emdb/structures/EMD-29586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29586 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29586 | HTTPS FTP |
-Related structure data
| Related structure data |  8fynMC  8fyoC  8fypC  8fyqC  8fyrC  8fysC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29586.map.gz / Format: CCP4 / Size: 14.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29586.map.gz / Format: CCP4 / Size: 14.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the human A2A adenosine receptor from plasma milled lamellae | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X: 0.4885 Å / Y: 0.4885 Å / Z: 0.48365 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 20 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : A2A BRIL Adenosine receptor
| Entire | Name: A2A BRIL Adenosine receptor |
|---|---|
| Components |
|
-Supramolecule #1: A2A BRIL Adenosine receptor
| Supramolecule | Name: A2A BRIL Adenosine receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41 KDa |
-Macromolecule #1: Adenosine receptor A2a,Soluble cytochrome b562
| Macromolecule | Name: Adenosine receptor A2a,Soluble cytochrome b562 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 49.974281 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDGAPPIMG SSVYITVELA IAVLAILGNV LVCWAVWLNS NLQNVTNYFV VSLAAADIAV GVLAIPFAI TISTGFCAAC HGCLFIACFV LVLTQSSIFS LLAIAIDRYI AIRIPLRYNG LVTGTRAKGI IAICWVLSFA I GLTPMLGW ...String: MKTIIALSYI FCLVFADYKD DDDGAPPIMG SSVYITVELA IAVLAILGNV LVCWAVWLNS NLQNVTNYFV VSLAAADIAV GVLAIPFAI TISTGFCAAC HGCLFIACFV LVLTQSSIFS LLAIAIDRYI AIRIPLRYNG LVTGTRAKGI IAICWVLSFA I GLTPMLGW NNCGQPKEGK NHSQGCGEGQ VACLFEDVVP MNYMVYFNFF ACVLVPLLLM LGVYLRIFLA ARRQLADLED NW ETLNDNL KVIEKADNAA QVKDALTKMR AAALDAQKAT PPKLEDKSPD SPEMKDFRHG FDILVGQIDD ALKLANEGKV KEA QAAAEQ LKTTRNAYIQ KYLERARSTL QKEVHAAKSL AIIVGLFALC WLPLHIINCF TFFCPDCSHA PLWLMYLAIV LSHT NSVVN PFIYAYRIRE FRQTFRKIIR SHVLRQQEPF KAHHHHHHHH HH UniProtKB: Adenosine receptor A2a, Soluble cytochrome b562, Adenosine receptor A2a |
-Macromolecule #2: 4-{2-[(7-amino-2-furan-2-yl[1,2,4]triazolo[1,5-a][1,3,5]triazin-5...
| Macromolecule | Name: 4-{2-[(7-amino-2-furan-2-yl[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl)amino]ethyl}phenol type: ligand / ID: 2 / Number of copies: 1 / Formula: ZMA |
|---|---|
| Molecular weight | Theoretical: 337.336 Da |
| Chemical component information | 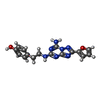 ChemComp-ZMA: |
-Macromolecule #3: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 3 / Number of copies: 3 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #4: OLEIC ACID
| Macromolecule | Name: OLEIC ACID / type: ligand / ID: 4 / Number of copies: 15 / Formula: OLA |
|---|---|
| Molecular weight | Theoretical: 282.461 Da |
| Chemical component information |  ChemComp-OLA: |
-Macromolecule #5: (2R)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate
| Macromolecule | Name: (2R)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate / type: ligand / ID: 5 / Number of copies: 5 / Formula: OLC |
|---|---|
| Molecular weight | Theoretical: 356.54 Da |
| Chemical component information |  ChemComp-OLC: |
-Macromolecule #6: (2S)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate
| Macromolecule | Name: (2S)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate / type: ligand / ID: 6 / Number of copies: 1 / Formula: OLB |
|---|---|
| Molecular weight | Theoretical: 356.54 Da |
| Chemical component information | 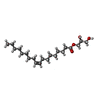 ChemComp-OLB: |
-Macromolecule #7: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 7 / Number of copies: 1 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #8: water
| Macromolecule | Name: water / type: ligand / ID: 8 / Number of copies: 174 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron crystallography |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Concentration | 25 mg/mL |
|---|---|
| Buffer | pH: 4.7 |
| Grid | Model: Quantifoil R2/2 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 10 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: LEICA PLUNGER |
| Details | Milled microcrystals |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 90.0 K |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Digitization - Dimensions - Width: 2048 pixel / Digitization - Dimensions - Height: 2048 pixel / Number grids imaged: 1 / Number real images: 1 / Number diffraction images: 840 / Average exposure time: 0.5 sec. / Average electron dose: 0.001 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION / Cs: 2.7 mm / Nominal defocus max: 0.0 µm / Nominal defocus min: 0.0 µm / Camera length: 1202 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN / Tilt angle: -30.0, 30.0 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Binned by 2 |
|---|---|
| Final reconstruction | Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 2.0 Å / Resolution method: DIFFRACTION PATTERN/LAYERLINES |
| Merging software list | Software - Name: AIMLESS |
| Crystallography statistics | Number intensities measured: 569407 / Number structure factors: 64974 / Fourier space coverage: 87.58 / R sym: 0.073 / R merge: 0.236 / Overall phase error: 30 / Overall phase residual: 0 / Phase error rejection criteria: None / High resolution: 0.87 Å / Shell - Shell ID: 1 / Shell - High resolution: 0.87 Å / Shell - Low resolution: 0.9 Å / Shell - Number structure factors: 2783 / Shell - Phase residual: 30 / Shell - Fourier space coverage: 37.64 / Shell - Multiplicity: 2.1 |
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL / Overall B value: 12.54 / Target criteria: Maximum likelihood |
|---|---|
| Output model |  PDB-8fyn: |
 Movie
Movie Controller
Controller






















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)




















