+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | KaiC-RS-S413E/S414E full dodecamer reconstruction | |||||||||
 Map data Map data | KaiC-RS-S413E/S414E full dodecamer sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | autokinase / CIRCADIAN CLOCK PROTEIN | |||||||||
| Biological species |  Cereibacter sphaeroides (bacteria) Cereibacter sphaeroides (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.22 Å | |||||||||
 Authors Authors | Padua RAP / Grant T / Pitsawong W / Hoemberger MS / Otten R / Bradshaw N / Grigorieff N / Kern D | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: From primordial clocks to circadian oscillators. Authors: Warintra Pitsawong / Ricardo A P Pádua / Timothy Grant / Marc Hoemberger / Renee Otten / Niels Bradshaw / Nikolaus Grigorieff / Dorothee Kern /  Abstract: Circadian rhythms play an essential part in many biological processes, and only three prokaryotic proteins are required to constitute a true post-translational circadian oscillator. The evolutionary ...Circadian rhythms play an essential part in many biological processes, and only three prokaryotic proteins are required to constitute a true post-translational circadian oscillator. The evolutionary history of the three Kai proteins indicates that KaiC is the oldest member and a central component of the clock. Subsequent additions of KaiB and KaiA regulate the phosphorylation state of KaiC for time synchronization. The canonical KaiABC system in cyanobacteria is well understood, but little is known about more ancient systems that only possess KaiBC. However, there are reports that they might exhibit a basic, hourglass-like timekeeping mechanism. Here we investigate the primordial circadian clock in Rhodobacter sphaeroides, which contains only KaiBC, to elucidate its inner workings despite missing KaiA. Using a combination of X-ray crystallography and cryogenic electron microscopy, we find a new dodecameric fold for KaiC, in which two hexamers are held together by a coiled-coil bundle of 12 helices. This interaction is formed by the carboxy-terminal extension of KaiC and serves as an ancient regulatory moiety that is later superseded by KaiA. A coiled-coil register shift between daytime and night-time conformations is connected to phosphorylation sites through a long-range allosteric network that spans over 140 Å. Our kinetic data identify the difference in the ATP-to-ADP ratio between day and night as the environmental cue that drives the clock. They also unravel mechanistic details that shed light on the evolution of self-sustained oscillators. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29509.map.gz emd_29509.map.gz | 188.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29509-v30.xml emd-29509-v30.xml emd-29509.xml emd-29509.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29509_fsc.xml emd_29509_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_29509.png emd_29509.png | 78.4 KB | ||
| Filedesc metadata |  emd-29509.cif.gz emd-29509.cif.gz | 5 KB | ||
| Others |  emd_29509_half_map_1.map.gz emd_29509_half_map_1.map.gz emd_29509_half_map_2.map.gz emd_29509_half_map_2.map.gz | 191.1 MB 191.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29509 http://ftp.pdbj.org/pub/emdb/structures/EMD-29509 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29509 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29509 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29509.map.gz / Format: CCP4 / Size: 206 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29509.map.gz / Format: CCP4 / Size: 206 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | KaiC-RS-S413E/S414E full dodecamer sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
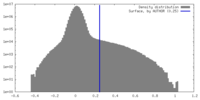
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: unsharpened half map
| File | emd_29509_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
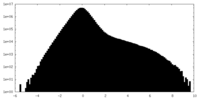
| Density Histograms |
-Half map: unsharpened half map
| File | emd_29509_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
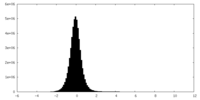
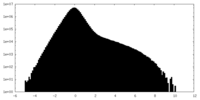
| Density Histograms |
- Sample components
Sample components
-Entire : Dodecamer of KaiC-RS-S413E/S414E
| Entire | Name: Dodecamer of KaiC-RS-S413E/S414E |
|---|---|
| Components |
|
-Supramolecule #1: Dodecamer of KaiC-RS-S413E/S414E
| Supramolecule | Name: Dodecamer of KaiC-RS-S413E/S414E / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Cereibacter sphaeroides (bacteria) Cereibacter sphaeroides (bacteria) |
| Molecular weight | Theoretical: 751 KDa |
-Macromolecule #1: KaiC-RS-S413E/S414E
| Macromolecule | Name: KaiC-RS-S413E/S414E / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Cereibacter sphaeroides (bacteria) Cereibacter sphaeroides (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: GAMGIGKSPT GIQGFDELTL GGLPTGRPSL VCGSAGCGKT LFASTFLING VRDHGEPGVF VTFEERPEDI VNNVASLGFE LDKLIEEEKI AIEHIAVDPS EVAEIGDYDL EGLFLRLELA IDTVGAKRVV LDTIESLFSA FSNPAILRAE IRRLFDWLKE RGLTTVITAE ...String: GAMGIGKSPT GIQGFDELTL GGLPTGRPSL VCGSAGCGKT LFASTFLING VRDHGEPGVF VTFEERPEDI VNNVASLGFE LDKLIEEEKI AIEHIAVDPS EVAEIGDYDL EGLFLRLELA IDTVGAKRVV LDTIESLFSA FSNPAILRAE IRRLFDWLKE RGLTTVITAE RGDGALTRQG LEEYVSDCVI LLDHRVENQI STRRLRIVKY RGTAHGTNEY PFLIDTDGFS VLPVSALGLL HQVHEERIAS GVPDLDAMMA GGGFFRGSSI LVSGVAGAGK SSLAAHFAAA ACARGERAMY FSFEEAADQA VRNMRSLGLD LGRWRDAGLL RFMATRPTFY SLEMHLAVIL REVMRFEPSV VVLDPISAFT ESGDRLEVQS MLLRIVDFLK NRGITGIFTH LAHSQNEATT DAGLEELMDG WVLMLNREVN GEFNRELYLL KARGMAHSNQ VREFLMSDRG ISLLPPHLGE GGALTGTARK AEEARLRRAE IERQTELGRL QQQIEQRRRR ARAQIEALEA ELQAEEIALK ALVESESAHE RQRLADADTL ARSRGNERFA DLLMNKGE |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)