+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2945 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of lysozyme solved by MicroED to 2.9 A | |||||||||
 Map data Map data | Diffraction data phased by molecular replacement (PDB ID: 3J4G) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | lysozyme | |||||||||
| Function / homology |  Function and homology information Function and homology informationLactose synthesis / Antimicrobial peptides / Neutrophil degranulation / beta-N-acetylglucosaminidase activity / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to Gram-positive bacterium ...Lactose synthesis / Antimicrobial peptides / Neutrophil degranulation / beta-N-acetylglucosaminidase activity / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to Gram-positive bacterium / defense response to bacterium / endoplasmic reticulum / extracellular space / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | electron crystallography / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Shi D / Nannenga BL / Iadanza MG / Gonen T | |||||||||
 Citation Citation |  Journal: Elife / Year: 2013 Journal: Elife / Year: 2013Title: Three-dimensional electron crystallography of protein microcrystals. Authors: Dan Shi / Brent L Nannenga / Matthew G Iadanza / Tamir Gonen /  Abstract: We demonstrate that it is feasible to determine high-resolution protein structures by electron crystallography of three-dimensional crystals in an electron cryo-microscope (CryoEM). Lysozyme ...We demonstrate that it is feasible to determine high-resolution protein structures by electron crystallography of three-dimensional crystals in an electron cryo-microscope (CryoEM). Lysozyme microcrystals were frozen on an electron microscopy grid, and electron diffraction data collected to 1.7 Å resolution. We developed a data collection protocol to collect a full-tilt series in electron diffraction to atomic resolution. A single tilt series contains up to 90 individual diffraction patterns collected from a single crystal with tilt angle increment of 0.1-1° and a total accumulated electron dose less than 10 electrons per angstrom squared. We indexed the data from three crystals and used them for structure determination of lysozyme by molecular replacement followed by crystallographic refinement to 2.9 Å resolution. This proof of principle paves the way for the implementation of a new technique, which we name 'MicroED', that may have wide applicability in structural biology. DOI: http://dx.doi.org/10.7554/eLife.01345.001. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2945.map.gz emd_2945.map.gz | 2.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2945-v30.xml emd-2945-v30.xml emd-2945.xml emd-2945.xml | 9.3 KB 9.3 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2945_3J4Gmask.png EMD-2945_3J4Gmask.png | 121.7 KB | ||
| Filedesc structureFactors |  emd_2945_sf.cif.gz emd_2945_sf.cif.gz | 46.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2945 http://ftp.pdbj.org/pub/emdb/structures/EMD-2945 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2945 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2945 | HTTPS FTP |
-Validation report
| Summary document |  emd_2945_validation.pdf.gz emd_2945_validation.pdf.gz | 363.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2945_full_validation.pdf.gz emd_2945_full_validation.pdf.gz | 362.7 KB | Display | |
| Data in XML |  emd_2945_validation.xml.gz emd_2945_validation.xml.gz | 4.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2945 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2945 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2945 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2945 | HTTPS FTP |
-Related structure data
| Related structure data |  3j4gMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2945.map.gz / Format: CCP4 / Size: 2.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2945.map.gz / Format: CCP4 / Size: 2.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Diffraction data phased by molecular replacement (PDB ID: 3J4G) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X: 0.713 Å / Y: 0.713 Å / Z: 0.685 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
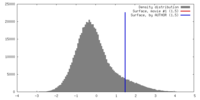
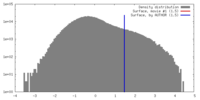
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Hen egg white lysozyme
| Entire | Name: Hen egg white lysozyme |
|---|---|
| Components |
|
-Supramolecule #1000: Hen egg white lysozyme
| Supramolecule | Name: Hen egg white lysozyme / type: sample / ID: 1000 / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 14.3 KDa |
-Macromolecule #1: Lysozyme C
| Macromolecule | Name: Lysozyme C / type: protein_or_peptide / ID: 1 / Name.synonym: Hen egg white lysozyme / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 14.3 KDa |
| Sequence | UniProtKB: Lysozyme C |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron crystallography |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Concentration | 200 mg/mL |
|---|---|
| Buffer | pH: 4.5 Details: 3.5M sodium chloride; 15% PEG 5,000; 50 mM sodium acetate |
| Grid | Details: glow discharged copper grid with holey carbon support |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 100 K / Instrument: FEI VITROBOT MARK IV / Method: blot approximately 10 seconds before plunging |
| Details | batch crystallization |
| Crystal formation | Details: batch crystallization |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 90 K / Max: 110 K / Average: 100 K |
| Details | selected area diffraction |
| Date | Jul 1, 2013 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Average electron dose: 0.1 e/Å2 / Camera length: 1500 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: DIFFRACTION |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN / Tilt angle min: -45 / Tilt angle max: 45 / Tilt series - Axis1 - Min angle: -45 ° / Tilt series - Axis1 - Max angle: 45 ° |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.9 Å / Resolution method: DIFFRACTION PATTERN/LAYERLINES |
|---|---|
| Crystal parameters | Unit cell - A: 77 Å / Unit cell - B: 77 Å / Unit cell - C: 37 Å / Unit cell - γ: 90 ° / Unit cell - α: 90 ° / Unit cell - β: 90 ° / Space group: P 43 21 2 |
 Movie
Movie Controller
Controller








 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)