+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Human PTH1R in complex with PTHrP and Gs | |||||||||||||||
 マップデータ マップデータ | Main map sharpened (resized to 0.85 angstrom/px) | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | GPCR / agonist / hormone / MEMBRANE PROTEIN | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of chondrocyte development / regulation of chondrocyte differentiation / parathyroid hormone receptor activity / cAMP metabolic process / Class B/2 (Secretin family receptors) / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding ...negative regulation of chondrocyte development / regulation of chondrocyte differentiation / parathyroid hormone receptor activity / cAMP metabolic process / Class B/2 (Secretin family receptors) / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding / bone mineralization / peptide hormone binding / PKA activation in glucagon signalling / developmental growth / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / hair follicle placode formation / epidermis development / chondrocyte differentiation / D1 dopamine receptor binding / bone resorption / intracellular transport / vascular endothelial cell response to laminar fluid shear stress / renal water homeostasis / activation of adenylate cyclase activity / cell maturation / Hedgehog 'off' state / adenylate cyclase-activating adrenergic receptor signaling pathway / cellular response to glucagon stimulus / regulation of insulin secretion / adenylate cyclase activator activity / trans-Golgi network membrane / skeletal system development / negative regulation of inflammatory response to antigenic stimulus / female pregnancy / hormone activity / bone development / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / platelet aggregation / G-protein beta/gamma-subunit complex binding / cognition / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / intracellular calcium ion homeostasis / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / sensory perception of smell / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / cell-cell signaling / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / regulation of gene expression / positive regulation of cold-induced thermogenesis / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / basolateral plasma membrane / 加水分解酵素; 酸無水物に作用; GTPに作用・細胞または細胞小器官の運動に関与 / G alpha (q) signalling events / in utero embryonic development / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell surface receptor signaling pathway 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||||||||
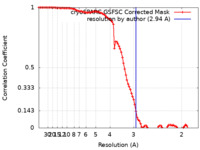
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.94 Å | |||||||||||||||
 データ登録者 データ登録者 | Cary BP / Belousoff MJ / Piper SJ / Wootten D / Sexton PM | |||||||||||||||
| 資金援助 |  オーストラリア, 4件 オーストラリア, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Structure / 年: 2023 ジャーナル: Structure / 年: 2023タイトル: Molecular insights into peptide agonist engagement with the PTH receptor. 著者: Brian P Cary / Elliot J Gerrard / Matthew J Belousoff / Madeleine M Fletcher / Yan Jiang / Isabella C Russell / Sarah J Piper / Denise Wootten / Patrick M Sexton /  要旨: The parathyroid hormone (PTH) 1 receptor (PTH1R) is a G protein-coupled receptor (GPCR) that regulates skeletal development and calcium homeostasis. Here, we describe cryo-EM structures of the PTH1R ...The parathyroid hormone (PTH) 1 receptor (PTH1R) is a G protein-coupled receptor (GPCR) that regulates skeletal development and calcium homeostasis. Here, we describe cryo-EM structures of the PTH1R in complex with fragments of the two hormones, PTH and PTH-related protein, the drug abaloparatide, as well as the engineered tool compounds, long-acting PTH (LA-PTH) and the truncated peptide, M-PTH(1-14). We found that the critical N terminus of each agonist engages the transmembrane bundle in a topologically similar fashion, reflecting similarities in measures of Gαs activation. The full-length peptides induce subtly different extracellular domain (ECD) orientations relative to the transmembrane domain. In the structure bound to M-PTH, the ECD is unresolved, demonstrating that the ECD is highly dynamic when unconstrained by a peptide. High resolutions enabled identification of water molecules near peptide and G protein binding sites. Our results illuminate the action of orthosteric agonists of the PTH1R. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_29284.map.gz emd_29284.map.gz | 85.3 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-29284-v30.xml emd-29284-v30.xml emd-29284.xml emd-29284.xml | 30.1 KB 30.1 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_29284_fsc.xml emd_29284_fsc.xml | 9.5 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_29284.png emd_29284.png | 107.1 KB | ||
| Filedesc metadata |  emd-29284.cif.gz emd-29284.cif.gz | 7.6 KB | ||
| その他 |  emd_29284_additional_1.map.gz emd_29284_additional_1.map.gz emd_29284_additional_2.map.gz emd_29284_additional_2.map.gz emd_29284_half_map_1.map.gz emd_29284_half_map_1.map.gz emd_29284_half_map_2.map.gz emd_29284_half_map_2.map.gz | 45.5 MB 774.5 KB 84.6 MB 84.6 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29284 http://ftp.pdbj.org/pub/emdb/structures/EMD-29284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29284 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_29284_validation.pdf.gz emd_29284_validation.pdf.gz | 842.7 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_29284_full_validation.pdf.gz emd_29284_full_validation.pdf.gz | 842.3 KB | 表示 | |
| XML形式データ |  emd_29284_validation.xml.gz emd_29284_validation.xml.gz | 17.9 KB | 表示 | |
| CIF形式データ |  emd_29284_validation.cif.gz emd_29284_validation.cif.gz | 22.9 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29284 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29284 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29284 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29284 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_29284.map.gz / 形式: CCP4 / 大きさ: 91.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_29284.map.gz / 形式: CCP4 / 大きさ: 91.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Main map sharpened (resized to 0.85 angstrom/px) | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: Main map usharpened (resized to 0.85 angstrom/px)
| ファイル | emd_29284_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
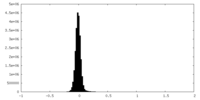
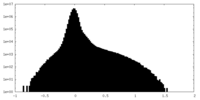
| 注釈 | Main map usharpened (resized to 0.85 angstrom/px) | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
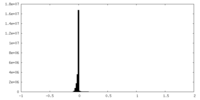
| 密度ヒストグラム |
-追加マップ: #1
| ファイル | emd_29284_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
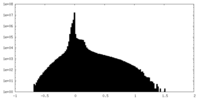
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_29284_half_map_1.map | ||||||||||||
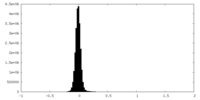
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
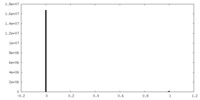
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_29284_half_map_2.map | ||||||||||||
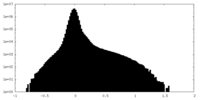
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
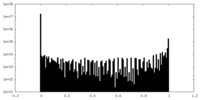
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : PTHrP bound to the PTH1R in complex with G protein
| 全体 | 名称: PTHrP bound to the PTH1R in complex with G protein |
|---|---|
| 要素 |
|
-超分子 #1: PTHrP bound to the PTH1R in complex with G protein
| 超分子 | 名称: PTHrP bound to the PTH1R in complex with G protein / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#6 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| 分子 | 名称: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 45.683434 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE ...文字列: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE YQLIDCAQYF LDKIDVIKQA DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGAQR DERRKWIQCF ND VTAIIFV VASSSYNMVI REDNQTNRLQ AALKLFDSIW NNKWLRDTSV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTT PEDATP EPGEDPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-分子 #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 37.413863 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...文字列: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 6.375332 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFR UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #4: Nanobody35
| 分子 | 名称: Nanobody35 / タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 13.885439 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-分子 #5: PTHrP[1-36]
| 分子 | 名称: PTHrP[1-36] / タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 4.269908 KDa |
| 配列 | 文字列: AVSEHQLLHD KGKSIQDLRR RFFLHHLIAE IHTAEI UniProtKB: Parathyroid hormone-related protein |
-分子 #6: Parathyroid hormone/parathyroid hormone-related peptide receptor
| 分子 | 名称: Parathyroid hormone/parathyroid hormone-related peptide receptor タイプ: protein_or_peptide / ID: 6 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 69.513758 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASG KLYPESEEDK EAPTGSRYRG RPCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE L VPGHNRTW ...文字列: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASG KLYPESEEDK EAPTGSRYRG RPCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE L VPGHNRTW ANYSECVKFL TNETREREVF DRLGMIYTVG YSVSLASLTV AVLILAYFRR LHCTRNYIHM HLFLSFMLRA VS IFVKDAV LYSGATLDEA ERLTEEELRA IAQAPPPPAT AAAGYAGCRV AVTFFLYFLA TNYYWILVEG LYLHSLIFMA FFS EKKYLW GFTVFGWGLP AVFVAVWVSV RATLANTGCW DLSSGNKKWI IQVPILASIV LNFILFINIV RVLATKLRET NAGR CDTRQ QYRKLLKSTL VLMPLFGVHY IVFMATPYTE VSGTLWQVQM HYEMLFNSFQ GFFVAIIYCF CNGEVQAEIK KSWSR WTLA LDFKRKARSG SSSYSYGPMV SHTSVTNVGP RVGLGLPLSP RLLPTATTNG HPQLPGHAKP GTPALETLET TPPAMA APK DDGFLNGSCS GLDEEASGPE RPPALLQEEW ETVMPAGLEV LFQGPHHHHH HHH UniProtKB: Parathyroid hormone/parathyroid hormone-related peptide receptor |
-分子 #7: water
| 分子 | 名称: water / タイプ: ligand / ID: 7 / コピー数: 10 / 式: HOH |
|---|---|
| 分子量 | 理論値: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 3.66 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.4 構成要素:
詳細: Buffer also contained 2 micromolar PTHrP peptide. | |||||||||||||||||||||
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: GOLD / メッシュ: 300 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 60 sec. / 前処理 - 雰囲気: AIR / 詳細: 20 mA current, negative polarity | |||||||||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | TFS GLACIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: FEI FALCON IV (4k x 4k) 撮影したグリッド数: 1 / 実像数: 4553 / 平均露光時間: 7.39 sec. / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 1.3 µm / 最小 デフォーカス(公称値): 0.6 µm |
| 試料ステージ | ホルダー冷却材: NITROGEN |
 ムービー
ムービー コントローラー
コントローラー
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)