[English] 日本語
 Yorodumi
Yorodumi- EMDB-29239: Local refinement of YrbE and MCE ring in Msmeg Mce1 transporter i... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Local refinement of YrbE and MCE ring in Msmeg Mce1 transporter in the absence of LucB (Map2b) | |||||||||
 Map data Map data | Constituent Map2b used to in composite Map2. Map was generated by locally refining Class2 particles that were re-centered and extracted with a smaller boxsize (360 px). | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Membrane protein complex / ABC transporter / Virulence factor / Lipid transport / MEMBRANE PROTEIN | |||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||
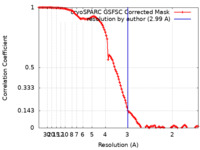
| Method | single particle reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||
 Authors Authors | Chen J / Bhabha G / Ekiert DC | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structure of an endogenous mycobacterial MCE lipid transporter. Authors: James Chen / Alice Fruhauf / Catherine Fan / Jackeline Ponce / Beatrix Ueberheide / Gira Bhabha / Damian C Ekiert /  Abstract: To replicate inside macrophages and cause tuberculosis, Mycobacterium tuberculosis must scavenge a variety of nutrients from the host. The mammalian cell entry (MCE) proteins are important virulence ...To replicate inside macrophages and cause tuberculosis, Mycobacterium tuberculosis must scavenge a variety of nutrients from the host. The mammalian cell entry (MCE) proteins are important virulence factors in M. tuberculosis, where they are encoded by large gene clusters and have been implicated in the transport of fatty acids and cholesterol across the impermeable mycobacterial cell envelope. Very little is known about how cargos are transported across this barrier, and it remains unclear how the approximately ten proteins encoded by a mycobacterial mce gene cluster assemble to transport cargo across the cell envelope. Here we report the cryo-electron microscopy (cryo-EM) structure of the endogenous Mce1 lipid-import machine of Mycobacterium smegmatis-a non-pathogenic relative of M. tuberculosis. The structure reveals how the proteins of the Mce1 system assemble to form an elongated ABC transporter complex that is long enough to span the cell envelope. The Mce1 complex is dominated by a curved, needle-like domain that appears to be unrelated to previously described protein structures, and creates a protected hydrophobic pathway for lipid transport across the periplasm. Our structural data revealed the presence of a subunit of the Mce1 complex, which we identified using a combination of cryo-EM and AlphaFold2, and name LucB. Our data lead to a structural model for Mce1-mediated lipid import across the mycobacterial cell envelope. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29239.map.gz emd_29239.map.gz | 168.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29239-v30.xml emd-29239-v30.xml emd-29239.xml emd-29239.xml | 29.7 KB 29.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29239_fsc.xml emd_29239_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_29239.png emd_29239.png | 94.4 KB | ||
| Others |  emd_29239_half_map_1.map.gz emd_29239_half_map_1.map.gz emd_29239_half_map_2.map.gz emd_29239_half_map_2.map.gz | 165.1 MB 165.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29239 http://ftp.pdbj.org/pub/emdb/structures/EMD-29239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29239 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29239.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29239.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Constituent Map2b used to in composite Map2. Map was generated by locally refining Class2 particles that were re-centered and extracted with a smaller boxsize (360 px). | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8255 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map A for Map2b
| File | emd_29239_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A for Map2b | ||||||||||||
| Projections & Slices |
| ||||||||||||
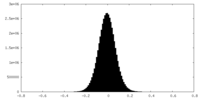
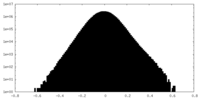
| Density Histograms |
-Half map: Half map B for Map2b
| File | emd_29239_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B for Map2b | ||||||||||||
| Projections & Slices |
| ||||||||||||
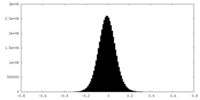
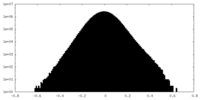
| Density Histograms |
- Sample components
Sample components
+Entire : Mce1 lipid transporter composed of Mce1 MCE proteins (Mce1ABCDEF)...
+Supramolecule #1: Mce1 lipid transporter composed of Mce1 MCE proteins (Mce1ABCDEF)...
+Macromolecule #1: Virulence factor Mce family protein Mce1A
+Macromolecule #2: Virulence factor Mce family protein Mce1B
+Macromolecule #3: Virulence factor Mce family protein Mce1C
+Macromolecule #4: Virulence factor Mce family protein Mce1D
+Macromolecule #5: Virulence factor Mce family protein Mce1E
+Macromolecule #6: Virulence factor Mce family protein Mce1F
+Macromolecule #7: ATP-binding protein MceG
+Macromolecule #8: ATP-binding protein MceG
+Macromolecule #9: ABC transporter permease protein YrbE1A
+Macromolecule #10: ABC transporter permease protein YrbE1B
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.7 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 50 mM Tris-HCl pH 7.5, 5 mM MgSO4, 150 mM NaCl, 1 mM DDM, 1 mM DTT | ||||||||||||||||||
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||||
| Details | This sample contains a mixture of MCE proteins endogenously purified from Mycobacterium smegmatis. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number grids imaged: 2 / Number real images: 43925 / Average exposure time: 2.0 sec. / Average electron dose: 60.0 e/Å2 Details: Images were collected in super resolution mode (nominal pixel size of 0.8255 A/pix after binning by 2). |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Model was initial fitted into the map using Chimera follow-by rigid body refinement in PHENIX. Models were further refined using PHENIX real-space refinement and then manually inspected in Coot. |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Cross-correlation coefficient |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)