[English] 日本語
 Yorodumi
Yorodumi- EMDB-29019: Alpha1/BetaB Heteromeric Glycine Receptor in 1 mM Glycine 20 uM I... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Alpha1/BetaB Heteromeric Glycine Receptor in 1 mM Glycine 20 uM Ivermectin State | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Glycine / Channel / Ivermectin / Pentameric / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationNeurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / glycine binding / ligand-gated monoatomic ion channel activity / cellular response to zinc ion / cellular response to ethanol / response to amino acid ...Neurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / glycine binding / ligand-gated monoatomic ion channel activity / cellular response to zinc ion / cellular response to ethanol / response to amino acid / chloride channel complex / monoatomic ion transport / chloride transmembrane transport / central nervous system development / cellular response to amino acid stimulus / transmembrane signaling receptor activity / intracellular protein localization / perikaryon / postsynaptic membrane / dendrite / zinc ion binding / membrane / plasma membrane / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | ||||||||||||
 Authors Authors | Gibbs E / Chakrapani S | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: High-throughput reprogramming of an NRPS condensation domain. Authors: Ines B Folger / Natália F Frota / Angelos Pistofidis / David L Niquille / Douglas A Hansen / T Martin Schmeing / Donald Hilvert /   Abstract: Engineered biosynthetic assembly lines could revolutionize the sustainable production of bioactive natural product analogs. Although yeast display is a proven, powerful tool for altering the ...Engineered biosynthetic assembly lines could revolutionize the sustainable production of bioactive natural product analogs. Although yeast display is a proven, powerful tool for altering the substrate specificity of gatekeeper adenylation domains in nonribosomal peptide synthetases (NRPSs), comparable strategies for other components of these megaenzymes have not been described. Here we report a high-throughput approach for engineering condensation (C) domains responsible for peptide elongation. We show that a 120-kDa NRPS module, displayed in functional form on yeast, can productively interact with an upstream module, provided in solution, to produce amide products tethered to the yeast surface. Using this system to screen a large C-domain library, we reprogrammed a surfactin synthetase module to accept a fatty acid donor, increasing catalytic efficiency for this noncanonical substrate >40-fold. Because C domains can function as selectivity filters in NRPSs, this methodology should facilitate the precision engineering of these molecular assembly lines. #2:  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Conformational transitions and allosteric modulation in a heteromeric glycine receptor Authors: Gibbs E / Klemm E / Seiferth D / Kumar A / Ilca SL / Biggin PC / Chakrapani S | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29019.map.gz emd_29019.map.gz | 51.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29019-v30.xml emd-29019-v30.xml emd-29019.xml emd-29019.xml | 28.5 KB 28.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29019_fsc.xml emd_29019_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_29019.png emd_29019.png | 84.4 KB | ||
| Masks |  emd_29019_msk_1.map emd_29019_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-29019.cif.gz emd-29019.cif.gz | 8 KB | ||
| Others |  emd_29019_half_map_1.map.gz emd_29019_half_map_1.map.gz emd_29019_half_map_2.map.gz emd_29019_half_map_2.map.gz | 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29019 http://ftp.pdbj.org/pub/emdb/structures/EMD-29019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29019 | HTTPS FTP |
-Related structure data
| Related structure data |  8fe1MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29019.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29019.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
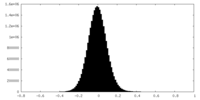
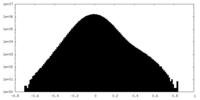
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_29019_msk_1.map emd_29019_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
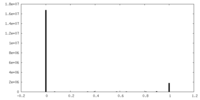
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29019_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
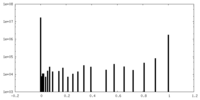
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_29019_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor
| Entire | Name: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor |
|---|---|
| Components |
|
-Supramolecule #1: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor
| Supramolecule | Name: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: Glycine receptor subunit alphaZ1
| Macromolecule | Name: Glycine receptor subunit alphaZ1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 52.537598 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN ...String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN FPMDVQTCIM QLESFGYTMN DLIFEWDEKG AVQVADGLTL PQFILKEEKD LRYCTKHYNT GKFTCIEARF HL ERQMGYY LIQMYIPSLL IVILSWVSFW INMDAAPARV GLGITTVLTM TTQSSGSRAS LPKVSYVKAI DIWMAVCLLF VFS ALLEYA AVNFIARQHK ELLRFQRRRR HLKEDEAGDG RFSFAAYGMG PACLQAKDGM AIKGNNNNAP TSTNPPEKTV EEMR KLFIS RAKRIDTVSR VAFPLVFLIF NIFYWITYKI IRSEDIHKQL VPRGSHHHHH HHH UniProtKB: Glycine receptor subunit alphaZ1 |
-Macromolecule #2: Glycine receptor beta subunit 2
| Macromolecule | Name: Glycine receptor beta subunit 2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.820281 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MKALKVIFML LIICLWMEGG FTKEKSAKKW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGS WSHPQFEKEN LYFQGEKSAK KGKKKGKQVY CPSQLSSEDL ARVPANSTSN ILNKLLITYD PRIRPNFKGI P VEDRVNIF ...String: MKALKVIFML LIICLWMEGG FTKEKSAKKW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGS WSHPQFEKEN LYFQGEKSAK KGKKKGKQVY CPSQLSSEDL ARVPANSTSN ILNKLLITYD PRIRPNFKGI P VEDRVNIF INSFGSIQET TMDYRVNIFL RQRWNDPRLR LPQDFKSDSL TVDPKMFKCL WKPDLFFANE KSANFHDVTQ EN ILLFIFR NGDVLISMRL SVTLSCPLDL TLFPMDTQRC KMQLESFGYT TDDLQFMWQS GDPVQMDEIA LPQFDIKQED IEY GNCTKY YAGTGYYTCV EVIFTLRRQV GFYMMGVYAP TLLIVVLSWL SFWINPDASA ARVPLGILSV LSLSSECTSL ASEL PKVSY VKAIDIWLIA CLLFGFASLV EYAVVQVMLN SPKLLEAERA KIATKEKAEG KTPAKNTING MGSTPIHVST LQVTE TRCK KVCTSKSDLR TNDFSIVGSL PRDFELSNFD CYGKPIEVGS AFSKSQAKNN KKPPPPKPVI PSAAKRIDLY ARALFP FSF LFFNVIYWSV YLENLYFQGT ETSQVAPA UniProtKB: Glycine receptor subunit beta |
-Macromolecule #3: GLYCINE
| Macromolecule | Name: GLYCINE / type: ligand / ID: 3 / Number of copies: 5 / Formula: GLY |
|---|---|
| Molecular weight | Theoretical: 75.067 Da |
| Chemical component information |  ChemComp-GLY: |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: (2aE,4E,5'S,6S,6'R,7S,8E,11R,13R,15S,17aR,20R,20aR,20bS)-6'-[(2S)...
| Macromolecule | Name: (2aE,4E,5'S,6S,6'R,7S,8E,11R,13R,15S,17aR,20R,20aR,20bS)-6'-[(2S)-butan-2-yl]-20,20b-dihydroxy-5',6,8,19-tetramethyl-17 -oxo-3',4',5',6,6',10,11,14,15,17,17a,20,20a,20b-tetradecahydro-2H,7H- ...Name: (2aE,4E,5'S,6S,6'R,7S,8E,11R,13R,15S,17aR,20R,20aR,20bS)-6'-[(2S)-butan-2-yl]-20,20b-dihydroxy-5',6,8,19-tetramethyl-17 -oxo-3',4',5',6,6',10,11,14,15,17,17a,20,20a,20b-tetradecahydro-2H,7H-spiro[11,15-methanofuro[4,3,2-pq][2,6]benzodioxacy clooctadecine-13,2'-pyran]-7-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-alpha-L-arabino-hexopyranosyl)-3-O-methyl-alpha-L-arabino-hexopyranoside type: ligand / ID: 5 / Number of copies: 5 / Formula: IVM |
|---|---|
| Molecular weight | Theoretical: 875.093 Da |
-Macromolecule #6: 1,2-DIMYRISTOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIMYRISTOYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 6 / Number of copies: 8 / Formula: PX4 |
|---|---|
| Molecular weight | Theoretical: 678.94 Da |
| Chemical component information | 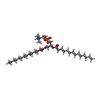 ChemComp-PX4: |
-Macromolecule #7: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 7 / Number of copies: 28 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #8: DECANE
| Macromolecule | Name: DECANE / type: ligand / ID: 8 / Number of copies: 1 / Formula: D10 |
|---|---|
| Molecular weight | Theoretical: 142.282 Da |
| Chemical component information |  ChemComp-D10: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | .1 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||||||||
| Details | Single Particles |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)