+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Yeast ATP synthase map in conformation-1 at pH 6 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | F-type ATP synthase / yeast / mitochondrial / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcristae formation / Mitochondrial protein degradation / mitochondrial proton-transporting ATP synthase complex assembly / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / mitochondrial nucleoid / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase ...cristae formation / Mitochondrial protein degradation / mitochondrial proton-transporting ATP synthase complex assembly / proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / mitochondrial nucleoid / proton motive force-driven mitochondrial ATP synthesis / proton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / proton transmembrane transport / ADP binding / mitochondrial intermembrane space / protein-containing complex assembly / mitochondrial inner membrane / lipid binding / ATP hydrolysis activity / mitochondrion / ATP binding / identical protein binding / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Sharma S / Patel H / Luo M / Mueller DM / Liao M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Conformational ensemble of yeast ATP synthase at low pH reveals unique intermediates and plasticity in F-F coupling. Authors: Stuti Sharma / Min Luo / Hiral Patel / David M Mueller / Maofu Liao /    Abstract: Mitochondrial adenosine triphosphate (ATP) synthase uses the proton gradient across the inner mitochondrial membrane to synthesize ATP. Structural and single molecule studies conducted mostly at ...Mitochondrial adenosine triphosphate (ATP) synthase uses the proton gradient across the inner mitochondrial membrane to synthesize ATP. Structural and single molecule studies conducted mostly at neutral or basic pH have provided details of the reaction mechanism of ATP synthesis. However, pH of the mitochondrial matrix is slightly acidic during hypoxia and pH-dependent conformational changes in the ATP synthase have been reported. Here we use single-particle cryo-EM to analyze the conformational ensemble of the yeast (Saccharomyces cerevisiae) ATP synthase at pH 6. Of the four conformations resolved in this study, three are reaction intermediates. In addition to canonical catalytic dwell and binding dwell structures, we identify two unique conformations with nearly identical positions of the central rotor but different catalytic site conformations. These structures provide new insights into the catalytic mechanism of the ATP synthase and highlight elastic coupling between the catalytic and proton translocating domains. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28809.map.gz emd_28809.map.gz | 49.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28809-v30.xml emd-28809-v30.xml emd-28809.xml emd-28809.xml | 30.4 KB 30.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28809.png emd_28809.png | 46.4 KB | ||
| Filedesc metadata |  emd-28809.cif.gz emd-28809.cif.gz | 7.9 KB | ||
| Others |  emd_28809_half_map_1.map.gz emd_28809_half_map_1.map.gz emd_28809_half_map_2.map.gz emd_28809_half_map_2.map.gz | 49.6 MB 49.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28809 http://ftp.pdbj.org/pub/emdb/structures/EMD-28809 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28809 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28809 | HTTPS FTP |
-Validation report
| Summary document |  emd_28809_validation.pdf.gz emd_28809_validation.pdf.gz | 923.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28809_full_validation.pdf.gz emd_28809_full_validation.pdf.gz | 923.4 KB | Display | |
| Data in XML |  emd_28809_validation.xml.gz emd_28809_validation.xml.gz | 11.1 KB | Display | |
| Data in CIF |  emd_28809_validation.cif.gz emd_28809_validation.cif.gz | 13.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28809 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28809 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28809 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28809 | HTTPS FTP |
-Related structure data
| Related structure data |  8f29MC  8f39C  8fkjC  8fl8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28809.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28809.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28809_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
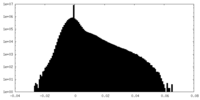
| Projections & Slices |
| ||||||||||||
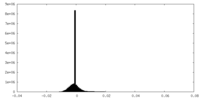
| Density Histograms |
-Half map: #1
| File | emd_28809_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
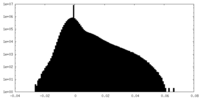
| Projections & Slices |
| ||||||||||||
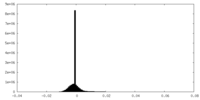
| Density Histograms |
- Sample components
Sample components
+Entire : ATP synthase
+Supramolecule #1: ATP synthase
+Macromolecule #1: ATP synthase subunit 5, mitochondrial
+Macromolecule #2: ATP synthase subunit gamma, mitochondrial
+Macromolecule #3: ATP synthase subunit delta, mitochondrial
+Macromolecule #4: ATP synthase subunit epsilon, mitochondrial
+Macromolecule #5: ATP synthase subunit 4, mitochondrial
+Macromolecule #6: ATP synthase subunit d, mitochondrial
+Macromolecule #7: ATP synthase subunit H, mitochondrial
+Macromolecule #8: ATP synthase subunit f, mitochondrial
+Macromolecule #9: ATP synthase subunit 9, mitochondrial
+Macromolecule #10: ATP synthase protein 8
+Macromolecule #11: ATP synthase subunit a
+Macromolecule #12: ATP synthase subunit J, mitochondrial
+Macromolecule #13: ATP synthase subunit alpha, mitochondrial
+Macromolecule #14: ATP synthase subunit beta, mitochondrial
+Macromolecule #15: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #16: MAGNESIUM ION
+Macromolecule #17: PHOSPHATE ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)