[English] 日本語
 Yorodumi
Yorodumi- EMDB-2873: Negative stain electron microscopy asymmetric reconstruction of h... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2873 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
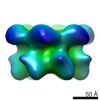
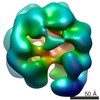
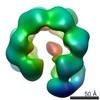
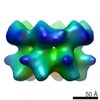
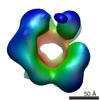
| Title | Negative stain electron microscopy asymmetric reconstruction of human minichromosome maintenance bound to a DNA substrate | |||||||||
 Map data Map data | Reconstruction of hMCM bound DNA | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | minichromosome maintenance / MCM2-7 / hMCM / DNA helicase / eukaryote | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 23.0 Å | |||||||||
 Authors Authors | Hesketh EL / Parker-Manuel RP / Chaban Y / Satti R / Coverley D / Orlova EV / Chong JPJ | |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2015 Journal: J Biol Chem / Year: 2015Title: DNA induces conformational changes in a recombinant human minichromosome maintenance complex. Authors: Emma L Hesketh / Richard P Parker-Manuel / Yuriy Chaban / Rabab Satti / Dawn Coverley / Elena V Orlova / James P J Chong /  Abstract: ATP-dependent DNA unwinding activity has been demonstrated for recombinant archaeal homohexameric minichromosome maintenance (MCM) complexes and their yeast heterohexameric counterparts, but in ...ATP-dependent DNA unwinding activity has been demonstrated for recombinant archaeal homohexameric minichromosome maintenance (MCM) complexes and their yeast heterohexameric counterparts, but in higher eukaryotes such as Drosophila, MCM-associated DNA helicase activity has been observed only in the context of a co-purified Cdc45-MCM-GINS complex. Here, we describe the production of the recombinant human MCM (hMCM) complex in Escherichia coli. This protein displays ATP hydrolysis activity and is capable of unwinding duplex DNA. Using single-particle asymmetric EM reconstruction, we demonstrate that recombinant hMCM forms a hexamer that undergoes a conformational change when bound to DNA. Recombinant hMCM produced without post-translational modifications is functional in vitro and provides an important tool for biochemical reconstitution of the human replicative helicase. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2873.map.gz emd_2873.map.gz | 1.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2873-v30.xml emd-2873-v30.xml emd-2873.xml emd-2873.xml | 8.7 KB 8.7 KB | Display Display |  EMDB header EMDB header |
| Images |  4.tif 4.tif | 187.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2873 http://ftp.pdbj.org/pub/emdb/structures/EMD-2873 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2873 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2873 | HTTPS FTP |
-Validation report
| Summary document |  emd_2873_validation.pdf.gz emd_2873_validation.pdf.gz | 189.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2873_full_validation.pdf.gz emd_2873_full_validation.pdf.gz | 188.7 KB | Display | |
| Data in XML |  emd_2873_validation.xml.gz emd_2873_validation.xml.gz | 5.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2873 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2873 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2873 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2873 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2873.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2873.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of hMCM bound DNA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
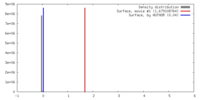
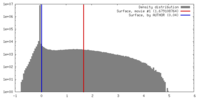
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Recombinant human mini chromosome maintenance complex bound to a ...
| Entire | Name: Recombinant human mini chromosome maintenance complex bound to a DNA substrate |
|---|---|
| Components |
|
-Supramolecule #1000: Recombinant human mini chromosome maintenance complex bound to a ...
| Supramolecule | Name: Recombinant human mini chromosome maintenance complex bound to a DNA substrate type: sample / ID: 1000 / Oligomeric state: heterohexamer / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 567 KDa |
-Macromolecule #1: human minichromosome maintenance (2-7) complex
| Macromolecule | Name: human minichromosome maintenance (2-7) complex / type: protein_or_peptide / ID: 1 / Name.synonym: hMCM, MCM2-7 / Number of copies: 1 / Oligomeric state: heterohexamer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Molecular weight | Theoretical: 567 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.999 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 25 mM HEPES pH 8, 200 mM sodium glutamate, 1 mM DTT, 0.1 mM AEBSF |
| Staining | Type: NEGATIVE / Details: Stained with methylamine tungstate, pH 7 |
| Grid | Details: Carbon coated grid |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Date | May 10, 2012 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 14 µm / Number real images: 72 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Calibrated magnification: 67000 / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
- Image processing
Image processing
| Details | Particles were selected using Boxer |
|---|---|
| CTF correction | Details: EACH MICROGRAPH |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 23.0 Å / Resolution method: OTHER / Software - Name: IMAGIC / Number images used: 1550 |
| Final two d classification | Number classes: 155 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera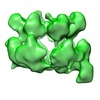


 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)