+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of S. aureus BlaR1 with C1 symmetry | |||||||||
 Map data Map data | Sharpened map from cryosparc local refinement. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Antibiotic resistance / beta-lactam antibiotics / MRSA / BlaR1 / MecR1 / cryo-EM / transmembrane signalling / SIGNALING PROTEIN | |||||||||
| Function / homology | : / Peptidase M56 / BlaR1 peptidase M56 / Penicillin-binding protein, transpeptidase / Penicillin binding protein transpeptidase domain / penicillin binding / Beta-lactamase/transpeptidase-like / membrane / BlaR1 Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.9 Å | |||||||||
 Authors Authors | Worrall LJ / Alexander JAN / Vuckovic M / Strynadka NCJ | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis of broad-spectrum β-lactam resistance in Staphylococcus aureus. Authors: J Andrew N Alexander / Liam J Worrall / Jinhong Hu / Marija Vuckovic / Nidhi Satishkumar / Raymond Poon / Solmaz Sobhanifar / Federico I Rosell / Joshua Jenkins / Daniel Chiang / Wesley A ...Authors: J Andrew N Alexander / Liam J Worrall / Jinhong Hu / Marija Vuckovic / Nidhi Satishkumar / Raymond Poon / Solmaz Sobhanifar / Federico I Rosell / Joshua Jenkins / Daniel Chiang / Wesley A Mosimann / Henry F Chambers / Mark Paetzel / Som S Chatterjee / Natalie C J Strynadka /   Abstract: Broad-spectrum β-lactam antibiotic resistance in Staphylococcus aureus is a global healthcare burden. In clinical strains, resistance is largely controlled by BlaR1, a receptor that senses β- ...Broad-spectrum β-lactam antibiotic resistance in Staphylococcus aureus is a global healthcare burden. In clinical strains, resistance is largely controlled by BlaR1, a receptor that senses β-lactams through the acylation of its sensor domain, inducing transmembrane signalling and activation of the cytoplasmic-facing metalloprotease domain. The metalloprotease domain has a role in BlaI derepression, inducing blaZ (β-lactamase PC1) and mecA (β-lactam-resistant cell-wall transpeptidase PBP2a) expression. Here, overcoming hurdles in isolation, we show that BlaR1 cleaves BlaI directly, as necessary for inactivation, with no requirement for additional components as suggested previously. Cryo-electron microscopy structures of BlaR1-the wild type and an autocleavage-deficient F284A mutant, with or without β-lactam-reveal a domain-swapped dimer that we suggest is critical to the stabilization of the signalling loops within. BlaR1 undergoes spontaneous autocleavage in cis between Ser283 and Phe284 and we describe the catalytic mechanism and specificity underlying the self and BlaI cleavage. The structures suggest that allosteric signalling emanates from β-lactam-induced exclusion of the prominent extracellular loop bound competitively in the sensor-domain active site, driving subsequent dynamic motions, including a shift in the sensor towards the membrane and accompanying changes in the zinc metalloprotease domain. We propose that this enhances the expulsion of autocleaved products from the active site, shifting the equilibrium to a state that is permissive of efficient BlaI cleavage. Collectively, this study provides a structure of a two-component signalling receptor that mediates action-in this case, antibiotic resistance-through the direct cleavage of a repressor. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28659.map.gz emd_28659.map.gz | 7.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28659-v30.xml emd-28659-v30.xml emd-28659.xml emd-28659.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28659_fsc.xml emd_28659_fsc.xml | 4.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_28659.png emd_28659.png | 93.4 KB | ||
| Filedesc metadata |  emd-28659.cif.gz emd-28659.cif.gz | 6 KB | ||
| Others |  emd_28659_half_map_1.map.gz emd_28659_half_map_1.map.gz emd_28659_half_map_2.map.gz emd_28659_half_map_2.map.gz | 7.4 MB 7.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28659 http://ftp.pdbj.org/pub/emdb/structures/EMD-28659 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28659 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28659 | HTTPS FTP |
-Related structure data
| Related structure data |  8exqMC  8expC  8exrC  8exsC  8extC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28659.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28659.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map from cryosparc local refinement. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.8047 Å | ||||||||||||||||||||||||||||||||||||
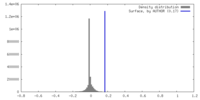
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map from cryosparc local refinement.
| File | emd_28659_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map from cryosparc local refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
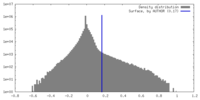
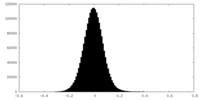
| Density Histograms |
-Half map: Half map from cryosparc local refinement.
| File | emd_28659_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map from cryosparc local refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
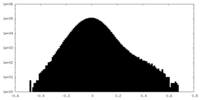
| Density Histograms |
- Sample components
Sample components
-Entire : Dimeric complex of S. aureus BlaR1
| Entire | Name: Dimeric complex of S. aureus BlaR1 |
|---|---|
| Components |
|
-Supramolecule #1: Dimeric complex of S. aureus BlaR1
| Supramolecule | Name: Dimeric complex of S. aureus BlaR1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 142 KDa |
-Macromolecule #1: Beta-lactam sensor/signal transducer BlaR1
| Macromolecule | Name: Beta-lactam sensor/signal transducer BlaR1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 71.269703 KDa |
| Recombinant expression | Organism:  Lactobacillus delbrueckii subsp. lactis (bacteria) Lactobacillus delbrueckii subsp. lactis (bacteria) |
| Sequence | String: MAKLLIMSIV SFCFIFLLLL FFRYILKRYF NYMLNYKVWY LTLLAGLIPF IPIKFSLFKF NNVNNQAPTV ESKSHDLNHN INTTKPIQE FATDIHKFNW DSIDNICTVI WIVLVIILSF KFLKALLYLK YLKKQSLYLN ENEKNKIDTI LFNHQYKKNI V IRKAETIQ ...String: MAKLLIMSIV SFCFIFLLLL FFRYILKRYF NYMLNYKVWY LTLLAGLIPF IPIKFSLFKF NNVNNQAPTV ESKSHDLNHN INTTKPIQE FATDIHKFNW DSIDNICTVI WIVLVIILSF KFLKALLYLK YLKKQSLYLN ENEKNKIDTI LFNHQYKKNI V IRKAETIQ SPITFWYGKY IILIPSSYFK SVIDKRLKYI ILHEYAHAKN RDTLHLIIFN IFSIIMSYNP LVHIVKRKII HD NEVEADR FVLNNINKNE FKTYAESIMD SVLNVPFFNK NILSHSFNGK KSLLKRRLIN IKEANLKKQS KLILIFICIF TFL LMVIQS QFLMGQSITD YNYKKPLHND YQILDKSKIF GSNSGSFVMY SMKKDKYYIY NEKESRKRYS PNSTYKIYLA MFGL DRHII NDENSRMSWN HKHYPFDAWN KEQDLNTAMQ NSVNWYFERI SDQIPKNYTA TQLKQLNYGN KNLGSYKSYW MEDSL KISN LEQVIVFKNM MEQNNHFSKK AKNQLSSSLL IKKNEKYELY GKTGTGIVNG KYNNGWFVGY VITNHDKYYF ATHLSD GKP SGKNAELISE KILKEMGVLN GQELALVPRG SSAHHHHHH UniProtKB: BlaR1 |
-Macromolecule #2: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20 mM HEPES, pH 7.5, 150 mM sodium chloride |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)