+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Class2 of the INO80-Hexasome complex | |||||||||
 Map data Map data | Class2,INO80-Hex,overall | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Chromatin Remodeler / hexasome / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationTTT Hsp90 cochaperone complex / R2TP complex / regulation of TOR signaling / Swr1 complex / Ino80 complex / telomere maintenance via recombination / ATP-dependent chromatin remodeler activity / regulation of metabolic process / box C/D snoRNP assembly / cellular response to stress ...TTT Hsp90 cochaperone complex / R2TP complex / regulation of TOR signaling / Swr1 complex / Ino80 complex / telomere maintenance via recombination / ATP-dependent chromatin remodeler activity / regulation of metabolic process / box C/D snoRNP assembly / cellular response to stress / 3'-5' DNA helicase activity / NuA4 histone acetyltransferase complex / chromosome, centromeric region / subtelomeric heterochromatin formation / enzyme regulator activity / DNA helicase activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / rRNA processing / histone binding / 5'-3' DNA helicase activity / transcription by RNA polymerase II / DNA helicase / chromosome, telomeric region / protein stabilization / chromatin remodeling / DNA repair / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / chromatin / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / DNA binding / ATP binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Wu H / Munoz E / Gourdet M / Cheng YF / Narlikar G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Reorientation of INO80 on hexasomes reveals basis for mechanistic versatility. Authors: Hao Wu / Elise N Muñoz / Laura J Hsieh / Un Seng Chio / Muryam A Gourdet / Geeta J Narlikar / Yifan Cheng /  Abstract: Unlike other chromatin remodelers, INO80 preferentially mobilizes hexasomes, which can form during transcription. Why INO80 prefers hexasomes over nucleosomes remains unclear. Here, we report ...Unlike other chromatin remodelers, INO80 preferentially mobilizes hexasomes, which can form during transcription. Why INO80 prefers hexasomes over nucleosomes remains unclear. Here, we report structures of INO80 bound to a hexasome or a nucleosome. INO80 binds the two substrates in substantially different orientations. On a hexasome, INO80 places its ATPase subunit, Ino80, at superhelical location -2 (SHL -2), in contrast to SHL -6 and SHL -7, as previously seen on nucleosomes. Our results suggest that INO80 action on hexasomes resembles action by other remodelers on nucleosomes such that Ino80 is maximally active near SHL -2. The SHL -2 position also plays a critical role for nucleosome remodeling by INO80. Overall, the mechanistic adaptations used by INO80 for preferential hexasome sliding imply that subnucleosomal particles play considerable regulatory roles. | |||||||||
| History |
|
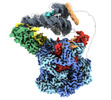
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28599.map.gz emd_28599.map.gz | 256.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28599-v30.xml emd-28599-v30.xml emd-28599.xml emd-28599.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28599.png emd_28599.png | 138.7 KB | ||
| Filedesc metadata |  emd-28599.cif.gz emd-28599.cif.gz | 6.9 KB | ||
| Others |  emd_28599_additional_1.map.gz emd_28599_additional_1.map.gz emd_28599_half_map_1.map.gz emd_28599_half_map_1.map.gz emd_28599_half_map_2.map.gz emd_28599_half_map_2.map.gz | 46.5 MB 290.5 MB 290.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28599 http://ftp.pdbj.org/pub/emdb/structures/EMD-28599 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28599 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28599 | HTTPS FTP |
-Related structure data
| Related structure data |  8etuMC  8etsC  8ettC  8etvC  8etwC  8eu2C  8eu9C  8eueC  8eufC  8eujC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28599.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28599.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class2,INO80-Hex,overall | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.835 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Class2, INO80-Hex,hex
| File | emd_28599_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class2, INO80-Hex,hex | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Class2,INO80-Hex,overall, half1
| File | emd_28599_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class2,INO80-Hex,overall, half1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Class2,INO80-Hex,overall, half2
| File | emd_28599_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class2,INO80-Hex,overall, half2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : INO80-Hex
| Entire | Name: INO80-Hex |
|---|---|
| Components |
|
-Supramolecule #1: INO80-Hex
| Supramolecule | Name: INO80-Hex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #5, #1-#2, #6 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Actin-related protein 5
| Macromolecule | Name: Actin-related protein 5 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 86.522047 KDa |
| Sequence | String: KAVVIDDPPL RQTPEPFDEQ SAYNPQSPIA IDFGSSKLRA GFVNHATPTH IFPNALTKFR DRKLNKNFTF VGNDTLLDQA VRSQSRSPF DGPFVTNWNL TEEILDYTFH HLGVVPDNGI PNPILLTERL ATVQSQRTNW YQILFETYNV PGVTFGIDSL F SFYNYNPS ...String: KAVVIDDPPL RQTPEPFDEQ SAYNPQSPIA IDFGSSKLRA GFVNHATPTH IFPNALTKFR DRKLNKNFTF VGNDTLLDQA VRSQSRSPF DGPFVTNWNL TEEILDYTFH HLGVVPDNGI PNPILLTERL ATVQSQRTNW YQILFETYNV PGVTFGIDSL F SFYNYNPS GNKTGLVISC GHEDTNVIPV VDGAGILTDA KRINWGGHQA VDYLNDLMAL KYPYFPTKMS YLQYETMYKD YC YVSRNYD EDIEKILTLE NLDTNDVVVE APFTEVLQPQ KTEEELRIQA EKRKETGKRL QEQARLKRME KLVQKQEEFE YFS KVRDQL IDEPKKKVLS VLQNAGFDDE RDFKKYLHSL EQSLKKAQMV EAEDDSHLDE MNEDKTAQKF DLLDIADEDL NEDQ IKEKR KQRFLKASQD ARQKAKEEKE RVAKEEEEKK LKEQQWRETD LNGWIKDKRL KLNKLIKRRK EKLKLRDEMK DRKSQ VSQN RMKNLASLAE DNVKQGAKRN RHQATIDNDP NDTFGANDED WLIYTDITQN PEAFEEALEY EYKDIVELER LLLEHD PNF TEEDTLEAQY DWRNSILHLF LRGPRPHDSE NIHEQHQMHL NVERIRVPEV IFQPTMGGQD QAGICELSET ILLKKFG SQ PGKLSQTSID MVNNVLITGG NAKVPGLKER IVKEFTGFLP TGTNITVNMS SDPSLDAWKG MAALARNEEQ YRKTVISK K EYEEYGPEYI KEHKLGNTKY FED UniProtKB: Actin-related protein 5 |
-Macromolecule #2: Chromatin-remodeling complex subunit IES6
| Macromolecule | Name: Chromatin-remodeling complex subunit IES6 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.881406 KDa |
| Sequence | String: ERLLFLRSVG ERNEIGFPSR FKSAHYKKPT RRHKSARQLI SDENKRINAL LTKANKAAES STAARRLVPK ATYFSVEAPP SIRPAKKYC DVTGLKGFYK SPTNNIRYHN AEIYQLIVKP MAPGVDQEYL KLRGANFVLK UniProtKB: Chromatin-remodeling complex subunit IES6 |
-Macromolecule #3: RuvB-like protein 1
| Macromolecule | Name: RuvB-like protein 1 / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.543805 KDa |
| Sequence | String: VTRTAAHTHI KGLGLDESGV AKRVEGGFVG QIEAREACGV IVDLIKAKKM SGRAILLAGG PSTGKTALAL AISQELGPKV PFCPLVGSE LYSVEVKKTE TLMENFRRAI GLRIKETKEV YEGEVTELTP EDAENPLGGY GKTISHVIVG LKSAKGTKTL R LDPTIYES ...String: VTRTAAHTHI KGLGLDESGV AKRVEGGFVG QIEAREACGV IVDLIKAKKM SGRAILLAGG PSTGKTALAL AISQELGPKV PFCPLVGSE LYSVEVKKTE TLMENFRRAI GLRIKETKEV YEGEVTELTP EDAENPLGGY GKTISHVIVG LKSAKGTKTL R LDPTIYES IQREKVSIGD VIYIEANTGA VKRVGRSDAY ATEFDLETEE YVPLPKGEVH KKKEIVQDVT LHDLDVANAR PQ GGQDVIS MMGQLLKPKK TEITEKLRQE VNKVVAKYID QGVAELIPGV LFIDEVNMLD IEIFTYLNKA LESNIAPVVV LAS NRGMTT VRGTEDVISP HGVPPDLIDR LLIVRTLPYD KDEIRTIIER RATVERLQVE SSALDLLATM GTETSLRYAL QLLA PCGIL AQTSNRKEIV VNDVNEAKLL FLDAKRSTKI LETSANYL UniProtKB: RuvB-like protein 1 |
-Macromolecule #4: RuvB-like protein 2
| Macromolecule | Name: RuvB-like protein 2 / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 50.153898 KDa |
| Sequence | String: KSLSLIAAHS HITGLGLDEN LQPRPTSEGM VGQLQARRAA GVILKMVQNG TIAGRAVLVA GPPSTGKTAL AMGVSQSLGK DVPFTAIAG SEIFSLELSK TEALTQAFRK SIGIKIKEET ELIEGEVVEI QIDRSITGGH KQGKLTIKTT DMETIYELGN K MIDGLTKE ...String: KSLSLIAAHS HITGLGLDEN LQPRPTSEGM VGQLQARRAA GVILKMVQNG TIAGRAVLVA GPPSTGKTAL AMGVSQSLGK DVPFTAIAG SEIFSLELSK TEALTQAFRK SIGIKIKEET ELIEGEVVEI QIDRSITGGH KQGKLTIKTT DMETIYELGN K MIDGLTKE KVLAGDVISI DKASGKITKL GRSFARSRDY DAMGADTRFV QCPEGELQKR KTVVHTVSLH EIDVINSRTQ GF LALFTGD TGEIRSEVRD QINTKVAEWK EEGKAEIVPG VLFIDEVHML DIECFSFINR ALEDEFAPIV MMATNRGVSK TRG TNYKSP HGLPLDLLDR SIIITTKSYN EQEIKTILSI RAQEEEVELS SDALDLLTKT GVETSLRYSS NLISVAQQIA MKRK NNTVE VEDVKRAYLL FLDSARSVKY VQENESQYID DQGNVQISIA KSADPDAMDT TE UniProtKB: RuvB-like protein 2 |
-Macromolecule #5: Chromatin-remodeling ATPase INO80
| Macromolecule | Name: Chromatin-remodeling ATPase INO80 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 54.985918 KDa |
| Sequence | String: IEIDVLCDLT QRQAKLYQVL KSQISTNYDA IENAATNDST SNSASNSGSD QNLINAVMQF RKVCNHPDLF ERADVDSPFS FTTFGKTTS MLTASVANNN SSVISNSNMN LSSMSSNNIS NGKFTDLIYS SRNPIKYSLP RLIYEDLILP NYNNDVDIAN K LKNVKFNI ...String: IEIDVLCDLT QRQAKLYQVL KSQISTNYDA IENAATNDST SNSASNSGSD QNLINAVMQF RKVCNHPDLF ERADVDSPFS FTTFGKTTS MLTASVANNN SSVISNSNMN LSSMSSNNIS NGKFTDLIYS SRNPIKYSLP RLIYEDLILP NYNNDVDIAN K LKNVKFNI FNPSTNYELC LFLSKLTGEP SLNEFFRVST TPLLKRVIER TNGPKNTDSL SFKTITQELL EVTRNAPSEG VM ASLLNVE KHAYEREYLN CIQRGYHPNV SAPPVTIEVL GSSHVTNSIN NELFDPLISQ ALSDIPAITQ YNMHVKKGIP VED FPKTGL FPEPLNKNFS SNISMPSMDR FITESAKLRK LDELLVKLKS EGHRVLIYFQ MTKMMDLMEE YLTYRQYNHI RLDG SSKLE DRRDLVHDWQ TNPEIFVFLL STRAGGLGIN LTAADTVIFY DSDWNPTIDS QAMDRAHRLG QTRQVTVYRL LVRGT IEER M UniProtKB: Chromatin-remodeling ATPase INO80 |
-Macromolecule #6: Ino eighty subunit 2
| Macromolecule | Name: Ino eighty subunit 2 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 3.531075 KDa |
| Sequence | String: FVKPRRPYNS EGMTRILRRY EEDLFCTF UniProtKB: Ino eighty subunit 2 |
-Macromolecule #7: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 7 / Number of copies: 6 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 67.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: -2.0 µm / Nominal defocus min: -1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 130147 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)