[English] 日本語
 Yorodumi
Yorodumi- EMDB-28583: CryoEM structure of GSDMB pore without transmembrane beta-barrel -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of GSDMB pore without transmembrane beta-barrel | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Pore-forming protein / GSDMB / pyroptosis / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytotoxic T cell pyroptotic cell death / wide pore channel activity / killing by host of symbiont cells / programmed cell death / cardiolipin binding / phosphatidylinositol-4-phosphate binding / phosphatidylserine binding / pyroptotic inflammatory response / phosphatidylinositol-4,5-bisphosphate binding / phospholipid binding ...cytotoxic T cell pyroptotic cell death / wide pore channel activity / killing by host of symbiont cells / programmed cell death / cardiolipin binding / phosphatidylinositol-4-phosphate binding / phosphatidylserine binding / pyroptotic inflammatory response / phosphatidylinositol-4,5-bisphosphate binding / phospholipid binding / defense response to Gram-negative bacterium / killing of cells of another organism / defense response to bacterium / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
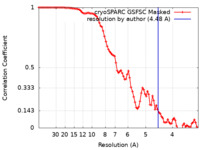
| Method | single particle reconstruction / cryo EM / Resolution: 4.48 Å | |||||||||
 Authors Authors | Wang C / Ruan J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Authors: Chengliang Wang / Sonia Shivcharan / Tian Tian / Skylar Wright / Danyang Ma / JengYih Chang / Kunpeng Li / Kangkang Song / Chen Xu / Vijay A Rathinam / Jianbin Ruan /  Abstract: Gasdermins (GSDMs) are pore-forming proteins that play critical roles in host defence through pyroptosis. Among GSDMs, GSDMB is unique owing to its distinct lipid-binding profile and a lack of ...Gasdermins (GSDMs) are pore-forming proteins that play critical roles in host defence through pyroptosis. Among GSDMs, GSDMB is unique owing to its distinct lipid-binding profile and a lack of consensus on its pyroptotic potential. Recently, GSDMB was shown to exhibit direct bactericidal activity through its pore-forming activity. Shigella, an intracellular, human-adapted enteropathogen, evades this GSDMB-mediated host defence by secreting IpaH7.8, a virulence effector that triggers ubiquitination-dependent proteasomal degradation of GSDMB. Here, we report the cryogenic electron microscopy structures of human GSDMB in complex with Shigella IpaH7.8 and the GSDMB pore. The structure of the GSDMB-IpaH7.8 complex identifies a motif of three negatively charged residues in GSDMB as the structural determinant recognized by IpaH7.8. Human, but not mouse, GSDMD contains this conserved motif, explaining the species specificity of IpaH7.8. The GSDMB pore structure shows the alternative splicing-regulated interdomain linker in GSDMB as a regulator of GSDMB pore formation. GSDMB isoforms with a canonical interdomain linker exhibit normal pyroptotic activity whereas other isoforms exhibit attenuated or no pyroptotic activity. Overall, this work sheds light on the molecular mechanisms of Shigella IpaH7.8 recognition and targeting of GSDMs and shows a structural determinant in GSDMB critical for its pyroptotic activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28583.map.gz emd_28583.map.gz | 59 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28583-v30.xml emd-28583-v30.xml emd-28583.xml emd-28583.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28583_fsc.xml emd_28583_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_28583.png emd_28583.png | 59.7 KB | ||
| Masks |  emd_28583_msk_1.map emd_28583_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-28583.cif.gz emd-28583.cif.gz | 6.3 KB | ||
| Others |  emd_28583_half_map_1.map.gz emd_28583_half_map_1.map.gz emd_28583_half_map_2.map.gz emd_28583_half_map_2.map.gz | 59.1 MB 59.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28583 http://ftp.pdbj.org/pub/emdb/structures/EMD-28583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28583 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28583 | HTTPS FTP |
-Validation report
| Summary document |  emd_28583_validation.pdf.gz emd_28583_validation.pdf.gz | 883.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28583_full_validation.pdf.gz emd_28583_full_validation.pdf.gz | 883.6 KB | Display | |
| Data in XML |  emd_28583_validation.xml.gz emd_28583_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_28583_validation.cif.gz emd_28583_validation.cif.gz | 20.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28583 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28583 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28583 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28583 | HTTPS FTP |
-Related structure data
| Related structure data |  8et1MC  8efpC  8et2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28583.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28583.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.66 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_28583_msk_1.map emd_28583_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_28583_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_28583_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GSDMB-pore
| Entire | Name: GSDMB-pore |
|---|---|
| Components |
|
-Supramolecule #1: GSDMB-pore
| Supramolecule | Name: GSDMB-pore / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: C24 symmetry |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Isoform 1 of Gasdermin-B
| Macromolecule | Name: Isoform 1 of Gasdermin-B / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.84825 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFSVFEEITR IVVKEMDAGG DMIAVRSLVD ADRFRCFHLV GEKRTFFGCR HYTTGLTLMD ILDTDGDKWL DELDSGLQGQ KAEFQILDN VDSTGELIVR LPKEITISGS FQGFHHQKIK ISENRISQQY LATLENRKLK RELPFSFRSI NTRENLYLVT E TLETVKEE ...String: MFSVFEEITR IVVKEMDAGG DMIAVRSLVD ADRFRCFHLV GEKRTFFGCR HYTTGLTLMD ILDTDGDKWL DELDSGLQGQ KAEFQILDN VDSTGELIVR LPKEITISGS FQGFHHQKIK ISENRISQQY LATLENRKLK RELPFSFRSI NTRENLYLVT E TLETVKEE TLKSDRQYKF WSQISQGHLS YKHKGQREVT IPPNRVLSYR VKQLVFPNKE TMSAGLDIHF RGKTKSFPEG KS LGSEDSR NMKEKLEDME SVLKDLTEEK RKDVLNSLAK CLGKEDIRQD LEQRVSEVLI SGELHMEDPD KPLLSSLFNA AGV LVEARA KAILDFLDAL LELSEEQQFV AEALEKGTLP LLKDQVKSVM EQNWDELASS PPDMDYDPEA RILCALYVVV SILL ELAEG PTSVSS UniProtKB: Gasdermin-B |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DIFFRACTION / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)