+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-2852 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Electron cryo-microscopy of mitochondrial ATP synthase dimers | |||||||||
 マップデータ マップデータ | Reconstruction of an ATP-synthase dimer | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | ATP-synthase / mitochondria / dimers / Polytomella | |||||||||
| 生物種 |  Polytomella (植物) Polytomella (植物) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 7.0 Å | |||||||||
 データ登録者 データ登録者 | Allegretti M / Klusch N / Mills DJ / Vonck J / Kuehlbrandt W / Davies KM | |||||||||
 引用 引用 |  ジャーナル: Nature / 年: 2015 ジャーナル: Nature / 年: 2015タイトル: Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase. 著者: Matteo Allegretti / Niklas Klusch / Deryck J Mills / Janet Vonck / Werner Kühlbrandt / Karen M Davies /  要旨: ATP, the universal energy currency of cells, is produced by F-type ATP synthases, which are ancient, membrane-bound nanomachines. F-type ATP synthases use the energy of a transmembrane ...ATP, the universal energy currency of cells, is produced by F-type ATP synthases, which are ancient, membrane-bound nanomachines. F-type ATP synthases use the energy of a transmembrane electrochemical gradient to generate ATP by rotary catalysis. Protons moving across the membrane drive a rotor ring composed of 8-15 c-subunits. A central stalk transmits the rotation of the c-ring to the catalytic F1 head, where a series of conformational changes results in ATP synthesis. A key unresolved question in this fundamental process is how protons pass through the membrane to drive ATP production. Mitochondrial ATP synthases form V-shaped homodimers in cristae membranes. Here we report the structure of a native and active mitochondrial ATP synthase dimer, determined by single-particle electron cryomicroscopy at 6.2 Å resolution. Our structure shows four long, horizontal membrane-intrinsic α-helices in the a-subunit, arranged in two hairpins at an angle of approximately 70° relative to the c-ring helices. It has been proposed that a strictly conserved membrane-embedded arginine in the a-subunit couples proton translocation to c-ring rotation. A fit of the conserved carboxy-terminal a-subunit sequence places the conserved arginine next to a proton-binding c-subunit glutamate. The map shows a slanting solvent-accessible channel that extends from the mitochondrial matrix to the conserved arginine. Another hydrophilic cavity on the lumenal membrane surface defines a direct route for the protons to an essential histidine-glutamate pair. Our results provide unique new insights into the structure and function of rotary ATP synthases and explain how ATP production is coupled to proton translocation. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_2852.map.gz emd_2852.map.gz | 9.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-2852-v30.xml emd-2852-v30.xml emd-2852.xml emd-2852.xml | 9 KB 9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
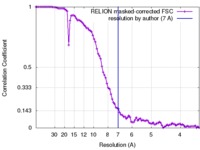
| FSC (解像度算出) |  emd_2852_fsc.xml emd_2852_fsc.xml | 9.1 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  500_500_EMD-2852.tif 500_500_EMD-2852.tif | 606 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2852 http://ftp.pdbj.org/pub/emdb/structures/EMD-2852 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2852 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2852 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_2852_validation.pdf.gz emd_2852_validation.pdf.gz | 236.6 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_2852_full_validation.pdf.gz emd_2852_full_validation.pdf.gz | 235.7 KB | 表示 | |
| XML形式データ |  emd_2852_validation.xml.gz emd_2852_validation.xml.gz | 11.1 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2852 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2852 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2852 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2852 | HTTPS FTP |
-関連構造データ
| 類似構造データ | |
|---|---|
| 電子顕微鏡画像生データ |  EMPIAR-10023 (タイトル: Electron cryo-microscopy of ATP synthase dimers from Polytomella sp. EMPIAR-10023 (タイトル: Electron cryo-microscopy of ATP synthase dimers from Polytomella sp.Data size: 4.1 TB Data #1: Multi-frame micrographs (1-24 frames) aligned by motion correction (Li et al 2013) [micrographs - multiframe]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_2852.map.gz / 形式: CCP4 / 大きさ: 62.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_2852.map.gz / 形式: CCP4 / 大きさ: 62.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Reconstruction of an ATP-synthase dimer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.77 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Polytomella ATP-synthase
| 全体 | 名称: Polytomella ATP-synthase |
|---|---|
| 要素 |
|
-超分子 #1000: Polytomella ATP-synthase
| 超分子 | 名称: Polytomella ATP-synthase / タイプ: sample / ID: 1000 / 集合状態: dimer / Number unique components: 1 |
|---|---|
| 分子量 | 理論値: 1.6 MDa |
-分子 #1: Mitochondrial F-type ATP-synthase
| 分子 | 名称: Mitochondrial F-type ATP-synthase / タイプ: protein_or_peptide / ID: 1 / 集合状態: Dimer / 組換発現: No |
|---|---|
| 由来(天然) | 生物種:  Polytomella (植物) / Organelle: Mitochondrion / 細胞中の位置: Cristae membrane Polytomella (植物) / Organelle: Mitochondrion / 細胞中の位置: Cristae membrane |
| 分子量 | 理論値: 1.6 MDa |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 0.5 mg/mL |
|---|---|
| 緩衝液 | pH: 8 詳細: 50nM TRIS/HCl pH 8.0, 1mM MgCl2, 20mM NaCl, DDM 0.05% |
| グリッド | 詳細: Quantifoil gold grid with thin carbon support, glow discharged for 2 minutes |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 75 % / チャンバー内温度: 113 K / 装置: FEI VITROBOT MARK I / 手法: Blot for 8-10 seconds before plunging |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI POLARA 300 |
|---|---|
| 温度 | 平均: 78 K |
| アライメント法 | Legacy - 非点収差: Objective lens astigmatism was corrected at 155,000 magnification |
| 日付 | 2014年1月30日 |
| 撮影 | カテゴリ: CCD フィルム・検出器のモデル: FEI FALCON II (4k x 4k) 実像数: 2829 / 平均電子線量: 80 e/Å2 詳細: Every image is a stack aligned by the motion correction software (Li et al., 2013) |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 104430 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.2 mm / 最大 デフォーカス(公称値): 5.0 µm / 最小 デフォーカス(公称値): 1.5 µm / 倍率(公称値): 59000 |
| 試料ステージ | 試料ホルダー: Nitrogen cooled / 試料ホルダーモデル: OTHER |
| 実験機器 |  モデル: Tecnai Polara / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)