[English] 日本語
 Yorodumi
Yorodumi- EMDB-28229: E. coli 70S ribosome with A-loop mutations U2554C and U2555C (30S... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli 70S ribosome with A-loop mutations U2554C and U2555C (30S Focus Refinement) | |||||||||
 Map data Map data | E. coli 70S ribosome with A-loop mutations U2554C and U2555C (30S Focus Refinement) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA / thermophile / A loop / RIBOSOME | |||||||||
| Biological species |  | |||||||||
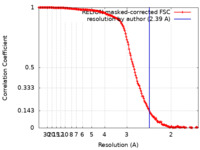
| Method | single particle reconstruction / cryo EM / Resolution: 2.39 Å | |||||||||
 Authors Authors | Nissley AJ / Penev PI / Watson ZL / Banfield JF / Cate JHD | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Rare ribosomal RNA sequences from archaea stabilize the bacterial ribosome. Authors: Amos J Nissley / Petar I Penev / Zoe L Watson / Jillian F Banfield / Jamie H D Cate /  Abstract: The ribosome serves as the universally conserved translator of the genetic code into proteins and supports life across diverse temperatures ranging from below freezing to above 120°C. Ribosomes are ...The ribosome serves as the universally conserved translator of the genetic code into proteins and supports life across diverse temperatures ranging from below freezing to above 120°C. Ribosomes are capable of functioning across this wide range of temperatures even though the catalytic site for peptide bond formation, the peptidyl transferase center, is nearly universally conserved. Here we find that Thermoproteota, a phylum of thermophilic Archaea, substitute cytidine for uridine at large subunit rRNA positions 2554 and 2555 (Escherichia coli numbering) in the A loop, immediately adjacent to the binding site for the 3'-end of A-site tRNA. We show by cryo-EM that E. coli ribosomes with uridine to cytidine mutations at these positions retain the proper fold and post-transcriptional modification of the A loop. Additionally, these mutations do not affect cellular growth, protect the large ribosomal subunit from thermal denaturation, and increase the mutational robustness of nucleotides in the peptidyl transferase center. This work identifies sequence variation across archaeal ribosomes in the peptidyl transferase center that likely confers stabilization of the ribosome at high temperatures and develops a stable mutant bacterial ribosome that can act as a scaffold for future ribosome engineering efforts. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28229.map.gz emd_28229.map.gz | 45.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28229-v30.xml emd-28229-v30.xml emd-28229.xml emd-28229.xml | 24.6 KB 24.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28229_fsc.xml emd_28229_fsc.xml | 15.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_28229.png emd_28229.png | 120.5 KB | ||
| Filedesc metadata |  emd-28229.cif.gz emd-28229.cif.gz | 4.6 KB | ||
| Others |  emd_28229_half_map_1.map.gz emd_28229_half_map_1.map.gz emd_28229_half_map_2.map.gz emd_28229_half_map_2.map.gz | 259.6 MB 259.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28229 http://ftp.pdbj.org/pub/emdb/structures/EMD-28229 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28229 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28229 | HTTPS FTP |
-Validation report
| Summary document |  emd_28229_validation.pdf.gz emd_28229_validation.pdf.gz | 977.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28229_full_validation.pdf.gz emd_28229_full_validation.pdf.gz | 977.3 KB | Display | |
| Data in XML |  emd_28229_validation.xml.gz emd_28229_validation.xml.gz | 23.6 KB | Display | |
| Data in CIF |  emd_28229_validation.cif.gz emd_28229_validation.cif.gz | 30.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28229 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28229 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28229 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28229 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28229.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28229.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli 70S ribosome with A-loop mutations U2554C and U2555C (30S Focus Refinement) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8279 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: E. coli 70S ribosome with A-loop mutations U2554C...
| File | emd_28229_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli 70S ribosome with A-loop mutations U2554C and U2555C (Half Map) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: E. coli 70S ribosome with A-loop mutations U2554C...
| File | emd_28229_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli 70S ribosome with A-loop mutations U2554C and U2555C (Half Map) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E. coli 70S ribosome with A-loop mutations U2554C and U2555C
| Entire | Name: E. coli 70S ribosome with A-loop mutations U2554C and U2555C |
|---|---|
| Components |
|
-Supramolecule #1: E. coli 70S ribosome with A-loop mutations U2554C and U2555C
| Supramolecule | Name: E. coli 70S ribosome with A-loop mutations U2554C and U2555C type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 850 KDa |
-Supramolecule #2: 50S Subunit
| Supramolecule | Name: 50S Subunit / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#5, #27-#54 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: 30S Subunit
| Supramolecule | Name: 30S Subunit / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #6-#26 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 12 sec. | ||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 38.8 |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)