+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human catalase | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Catalase / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to amitrole / response to phenylpropanoid / aminoacylase activity / catalase complex / hemoglobin metabolic process / response to inactivity / cellular detoxification of hydrogen peroxide / response to ozone / oxidoreductase activity, acting on peroxide as acceptor / response to L-ascorbic acid ...response to amitrole / response to phenylpropanoid / aminoacylase activity / catalase complex / hemoglobin metabolic process / response to inactivity / cellular detoxification of hydrogen peroxide / response to ozone / oxidoreductase activity, acting on peroxide as acceptor / response to L-ascorbic acid / catalase / response to fatty acid / response to light intensity / UV protection / catalase activity / response to vitamin A / peroxisomal membrane / ureteric bud development / triglyceride metabolic process / response to vitamin E / Detoxification of Reactive Oxygen Species / antioxidant activity / peroxisomal matrix / positive regulation of cell division / response to hyperoxia / Mitochondrial unfolded protein response (UPRmt) / response to cadmium ion / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / cholesterol metabolic process / aerobic respiration / response to reactive oxygen species / response to activity / hydrogen peroxide catabolic process / Peroxisomal protein import / response to hydrogen peroxide / response to insulin / response to lead ion / cellular response to growth factor stimulus / osteoblast differentiation / peroxisome / NADP binding / response to estradiol / response to ethanol / secretory granule lumen / ficolin-1-rich granule lumen / response to hypoxia / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / response to xenobiotic stimulus / focal adhesion / intracellular membrane-bounded organelle / heme binding / Neutrophil degranulation / negative regulation of apoptotic process / enzyme binding / protein homodimerization activity / protein-containing complex / mitochondrion / extracellular exosome / extracellular region / metal ion binding / identical protein binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
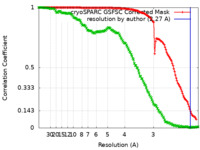
| Method | single particle reconstruction / cryo EM / Resolution: 2.27 Å | |||||||||
 Authors Authors | Su CC / Lyu M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of human catalase Authors: Su C-C / Yu E | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28225.map.gz emd_28225.map.gz | 230 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28225-v30.xml emd-28225-v30.xml emd-28225.xml emd-28225.xml | 16.8 KB 16.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28225_fsc.xml emd_28225_fsc.xml emd_28225_fsc_2.xml emd_28225_fsc_2.xml | 13.1 KB 18.1 KB | Display Display |  FSC data file FSC data file |
| Images |  emd_28225.png emd_28225.png | 79 KB | ||
| Filedesc metadata |  emd-28225.cif.gz emd-28225.cif.gz | 5.7 KB | ||
| Others |  emd_28225_additional_1.map.gz emd_28225_additional_1.map.gz emd_28225_half_map_1.map.gz emd_28225_half_map_1.map.gz emd_28225_half_map_2.map.gz emd_28225_half_map_2.map.gz | 123.5 MB 226.6 MB 226.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28225 http://ftp.pdbj.org/pub/emdb/structures/EMD-28225 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28225 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28225 | HTTPS FTP |
-Validation report
| Summary document |  emd_28225_validation.pdf.gz emd_28225_validation.pdf.gz | 847.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28225_full_validation.pdf.gz emd_28225_full_validation.pdf.gz | 847 KB | Display | |
| Data in XML |  emd_28225_validation.xml.gz emd_28225_validation.xml.gz | 22 KB | Display | |
| Data in CIF |  emd_28225_validation.cif.gz emd_28225_validation.cif.gz | 28.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28225 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28225 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28225 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28225 | HTTPS FTP |
-Related structure data
| Related structure data |  8el9MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28225.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28225.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Unsharpen map
| File | emd_28225_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpen map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_28225_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_28225_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : H6PD
| Entire | Name: H6PD |
|---|---|
| Components |
|
-Supramolecule #1: H6PD
| Supramolecule | Name: H6PD / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Catalase
| Macromolecule | Name: Catalase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: catalase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 59.836996 KDa |
| Sequence | String: MADSRDPASD QMQHWKEQRA AQKADVLTTG AGNPVGDKLN VITVGPRGPL LVQDVVFTDE MAHFDRERIP ERVVHAKGAG AFGYFEVTH DITKYSKAKV FEHIGKKTPI AVRFSTVAGE SGSADTVRDP RGFAVKFYTE DGNWDLVGNN TPIFFIRDPI L FPSFIHSQ ...String: MADSRDPASD QMQHWKEQRA AQKADVLTTG AGNPVGDKLN VITVGPRGPL LVQDVVFTDE MAHFDRERIP ERVVHAKGAG AFGYFEVTH DITKYSKAKV FEHIGKKTPI AVRFSTVAGE SGSADTVRDP RGFAVKFYTE DGNWDLVGNN TPIFFIRDPI L FPSFIHSQ KRNPQTHLKD PDMVWDFWSL RPESLHQVSF LFSDRGIPDG HRHMNGYGSH TFKLVNANGE AVYCKFHYKT DQ GIKNLSV EDAARLSQED PDYGIRDLFN AIATGKYPSW TFYIQVMTFN QAETFPFNPF DLTKVWPHKD YPLIPVGKLV LNR NPVNYF AEVEQIAFDP SNMPPGIEAS PDKMLQGRLF AYPDTHRHRL GPNYLHIPVN CPYRARVANY QRDGPMCMQD NQGG APNYY PNSFGAPEQQ PSALEHSIQY SGEVRRFNTA NDDNVTQVRA FYVNVLNEEQ RKRLCENIAG HLKDAQIFIQ KKAVK NFTE VHPDYGSHIQ ALLDKYNAEK PKNAIHTFVQ SGSHLAAREK ANL UniProtKB: Catalase |
-Macromolecule #2: PROTOPORPHYRIN IX CONTAINING FE
| Macromolecule | Name: PROTOPORPHYRIN IX CONTAINING FE / type: ligand / ID: 2 / Number of copies: 4 / Formula: HEM |
|---|---|
| Molecular weight | Theoretical: 616.487 Da |
| Chemical component information |  ChemComp-HEM: |
-Macromolecule #3: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE
| Macromolecule | Name: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE type: ligand / ID: 3 / Number of copies: 4 / Formula: NDP |
|---|---|
| Molecular weight | Theoretical: 745.421 Da |
| Chemical component information |  ChemComp-NDP: |
-Macromolecule #4: water
| Macromolecule | Name: water / type: ligand / ID: 4 / Number of copies: 1015 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | This is from a heterogeneous and impure protein sample. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 29.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



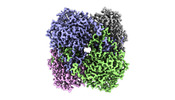











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)