+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HnRNPA2 D290V LCD PM3 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Amyloid / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationmiRNA transport / RNA transport / positive regulation of telomere maintenance via telomere lengthening / DNA geometric change / primary miRNA processing / N6-methyladenosine-containing RNA reader activity / single-stranded telomeric DNA binding / G-rich strand telomeric DNA binding / miRNA binding / Processing of Capped Intron-Containing Pre-mRNA ...miRNA transport / RNA transport / positive regulation of telomere maintenance via telomere lengthening / DNA geometric change / primary miRNA processing / N6-methyladenosine-containing RNA reader activity / single-stranded telomeric DNA binding / G-rich strand telomeric DNA binding / miRNA binding / Processing of Capped Intron-Containing Pre-mRNA / mRNA transport / mRNA export from nucleus / Cajal body / DNA polymerase binding / catalytic step 2 spliceosome / mRNA Splicing - Major Pathway / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / mRNA 3'-UTR binding / spliceosomal complex / molecular condensate scaffold activity / mRNA splicing, via spliceosome / nuclear matrix / mRNA processing / chromosome, telomeric region / ribonucleoprotein complex / RNA binding / extracellular exosome / nucleoplasm / identical protein binding / membrane / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Eisenberg DS / Lu J / Ge P / Boyer DR | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: Cryo-EM structures of the D290V mutant of the hnRNPA2 low-complexity domain suggests how D290V affects phase separation and aggregation. Authors: Jiahui Lu / Peng Ge / Michael R Sawaya / Michael P Hughes / David R Boyer / Qin Cao / Romany Abskharon / Duilio Cascio / Einav Tayeb-Fligelman / David S Eisenberg /   Abstract: Heterogeneous nuclear ribonucleoprotein A2 (hnRNPA2) is a human ribonucleoprotein that transports RNA to designated locations for translation via its ability to phase separate. Its mutated form, ...Heterogeneous nuclear ribonucleoprotein A2 (hnRNPA2) is a human ribonucleoprotein that transports RNA to designated locations for translation via its ability to phase separate. Its mutated form, D290V, is implicated in multisystem proteinopathy known to afflict two families, mainly with myopathy and Paget's disease of bone. Here, we investigate this mutant form of hnRNPA2 by determining cryo-EM structures of the recombinant D290V low complexity domain. We find that the mutant form of hnRNPA2 differs from the WT fibrils in four ways. In contrast to the WT fibrils, the PY-nuclear localization signals in the fibril cores of all three mutant polymorphs are less accessible to chaperones. Also, the mutant fibrils are more stable than WT fibrils as judged by phase separation, thermal stability, and energetic calculations. Similar to other pathogenic amyloids, the mutant fibrils are polymorphic. Thus, these structures offer evidence to explain how a D-to-V missense mutation diverts the assembly of reversible, functional amyloid-like fibrils into the assembly of pathogenic amyloid, and may shed light on analogous conversions occurring in other ribonucleoproteins that lead to neurological diseases such as amyotrophic lateral sclerosis and frontotemporal dementia. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28014.map.gz emd_28014.map.gz | 7.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28014-v30.xml emd-28014-v30.xml emd-28014.xml emd-28014.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28014.png emd_28014.png | 33.2 KB | ||
| Filedesc metadata |  emd-28014.cif.gz emd-28014.cif.gz | 5.1 KB | ||
| Others |  emd_28014_half_map_1.map.gz emd_28014_half_map_1.map.gz emd_28014_half_map_2.map.gz emd_28014_half_map_2.map.gz | 19.8 MB 19.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28014 http://ftp.pdbj.org/pub/emdb/structures/EMD-28014 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28014 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28014 | HTTPS FTP |
-Related structure data
| Related structure data |  8ec7MC  8du2C  8duwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28014.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28014.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.078 Å | ||||||||||||||||||||||||||||||||||||
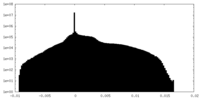
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28014_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
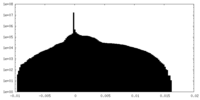
| Density Histograms |
-Half map: #1
| File | emd_28014_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
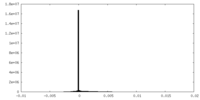
| Density Histograms |
- Sample components
Sample components
-Entire : Fibrils of hnRNPA2 D290V LCD PM3
| Entire | Name: Fibrils of hnRNPA2 D290V LCD PM3 |
|---|---|
| Components |
|
-Supramolecule #1: Fibrils of hnRNPA2 D290V LCD PM3
| Supramolecule | Name: Fibrils of hnRNPA2 D290V LCD PM3 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Heterogeneous nuclear ribonucleoproteins A2/B1
| Macromolecule | Name: Heterogeneous nuclear ribonucleoproteins A2/B1 / type: protein_or_peptide / ID: 1 / Number of copies: 15 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.391775 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQEVQSSRSG RGGNFGFGDS RGGGGNFGPG PGSNFRGGSD GYGSGRGFGD GYNGYGGGPG GGNFGGSPGY GGGRGGYGGG GPGYGNQGG GYGGGYDNYG GGNYGSGNYN VFGNYNQQPS NYGPMKSGNF GGSRNMGGPY GGGNYGPGGS GGSGGYGGRS R Y UniProtKB: Heterogeneous nuclear ribonucleoproteins A2/B1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 36.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 4.88 Å Applied symmetry - Helical parameters - Δ&Phi: -0.72 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 16114 |
|---|---|
| Startup model | Type of model: OTHER / Details: Gaussian blob |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller











 Y (Sec.)
Y (Sec.) X (Row.)
X (Row.) Z (Col.)
Z (Col.)