[English] 日本語
 Yorodumi
Yorodumi- EMDB-27893: The closed C1-state mouse TRPM8 structure in complex with PI(4,5)P2 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The closed C1-state mouse TRPM8 structure in complex with PI(4,5)P2 | |||||||||
 Map data Map data | Full map, sharpened with B-factor -10 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPM8 / menthol receptor / cold receptor / PI(4 / 5)P2 / cooling agonists / temperature sensing / ion channel / sensory transduction / transient receptor potential ion channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationTRP channels / thermoception / response to temperature stimulus / monoatomic ion channel activity / plasma membrane raft / response to cold / calcium channel activity / intracellular calcium ion homeostasis / calcium ion transport / positive regulation of cold-induced thermogenesis ...TRP channels / thermoception / response to temperature stimulus / monoatomic ion channel activity / plasma membrane raft / response to cold / calcium channel activity / intracellular calcium ion homeostasis / calcium ion transport / positive regulation of cold-induced thermogenesis / membrane raft / external side of plasma membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
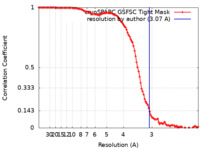
| Method | single particle reconstruction / cryo EM / Resolution: 3.07 Å | |||||||||
 Authors Authors | Yin Y / Zhang F / Feng S / Butay KJ / Borgnia MJ / Im W / Lee S-Y | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Activation mechanism of the mouse cold-sensing TRPM8 channel by cooling agonist and PIP. Authors: Ying Yin / Feng Zhang / Shasha Feng / Kevin John Butay / Mario J Borgnia / Wonpil Im / Seok-Yong Lee /  Abstract: The transient receptor potential melastatin 8 (TRPM8) channel is the primary molecular transducer responsible for the cool sensation elicited by menthol and cold in mammals. TRPM8 activation is ...The transient receptor potential melastatin 8 (TRPM8) channel is the primary molecular transducer responsible for the cool sensation elicited by menthol and cold in mammals. TRPM8 activation is controlled by cooling compounds together with the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP). Our knowledge of cold sensation and the therapeutic potential of TRPM8 for neuroinflammatory diseases and pain will be enhanced by understanding the structural basis of cooling agonist- and PIP-dependent TRPM8 activation. We present cryo-electron microscopy structures of mouse TRPM8 in closed, intermediate, and open states along the ligand- and PIP-dependent gating pathway. Our results uncover two discrete agonist sites, state-dependent rearrangements in the gate positions, and a disordered-to-ordered transition of the gate-forming S6-elucidating the molecular basis of chemically induced cool sensation in mammals. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27893.map.gz emd_27893.map.gz | 64.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27893-v30.xml emd-27893-v30.xml emd-27893.xml emd-27893.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27893_fsc.xml emd_27893_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_27893.png emd_27893.png | 68.4 KB | ||
| Masks |  emd_27893_msk_1.map emd_27893_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27893.cif.gz emd-27893.cif.gz | 6.8 KB | ||
| Others |  emd_27893_half_map_1.map.gz emd_27893_half_map_1.map.gz emd_27893_half_map_2.map.gz emd_27893_half_map_2.map.gz | 115.5 MB 115.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27893 http://ftp.pdbj.org/pub/emdb/structures/EMD-27893 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27893 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27893 | HTTPS FTP |
-Related structure data
| Related structure data |  8e4nMC  8e4lC  8e4mC  8e4oC  8e4pC  8e4qC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27893.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27893.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Full map, sharpened with B-factor -10 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27893_msk_1.map emd_27893_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_27893_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_27893_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Transient receptor potential cation channel subfamily M member 8
| Entire | Name: Transient receptor potential cation channel subfamily M member 8 |
|---|---|
| Components |
|
-Supramolecule #1: Transient receptor potential cation channel subfamily M member 8
| Supramolecule | Name: Transient receptor potential cation channel subfamily M member 8 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential cation channel subfamily M member 8
| Macromolecule | Name: Transient receptor potential cation channel subfamily M member 8 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 131.548312 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MASFEGARLS MRSRRNGTMG STRTLYSSVS RSTDVSYSDS DLVNFIQANF KKRECVFFTR DSKAMENICK CGYAQSQHIE GTQINQNEK WNYKKHTKEF PTDAFGDIQF ETLGKKGKYL RLSCDTDSET LYELLTQHWH LKTPNLVISV TGGAKNFALK P RMRKIFSR ...String: MASFEGARLS MRSRRNGTMG STRTLYSSVS RSTDVSYSDS DLVNFIQANF KKRECVFFTR DSKAMENICK CGYAQSQHIE GTQINQNEK WNYKKHTKEF PTDAFGDIQF ETLGKKGKYL RLSCDTDSET LYELLTQHWH LKTPNLVISV TGGAKNFALK P RMRKIFSR LIYIAQSKGA WILTGGTHYG LMKYIGEVVR DNTISRNSEE NIVAIGIAAW GMVSNRDTLI RSCDDEGHFS AQ YIMDDFT RDPLYILDNN HTHLLLVDNG CHGHPTVEAK LRNQLEKYIS ERTSQDSNYG GKIPIVCFAQ GGGRETLKAI NTS VKSKIP CVVVEGSGQI ADVIASLVEV EDVLTSSMVK EKLVRFLPRT VSRLPEEEIE SWIKWLKEIL ESSHLLTVIK MEEA GDEIV SNAISYALYK AFSTNEQDKD NWNGQLKLLL EWNQLDLASD EIFTNDRRWE SADLQEVMFT ALIKDRPKFV RLFLE NGLN LQKFLTNEVL TELFSTHFST LVYRNLQIAK NSYNDALLTF VWKLVANFRR SFWKEDRSSR EDLDVELHDA SLTTRH PLQ ALFIWAILQN KKELSKVIWE QTKGCTLAAL GASKLLKTLA KVKNDINAAG ESEELANEYE TRAVELFTEC YSNDEDL AE QLLVYSCEAW GGSNCLELAV EATDQHFIAQ PGVQNFLSKQ WYGEISRDTK NWKIILCLFI IPLVGCGLVS FRKKPIDK H KKLLWYYVAF FTSPFVVFSW NVVFYIAFLL LFAYVLLMDF HSVPHTPELI LYALVFVLFC DEVRQWYMNG VNYFTDLWN VMDTLGLFYF IAGIVFRLHS SNKSSLYSGR VIFCLDYIIF TLRLIHIFTV SRNLGPKIIM LQRMLIDVFF FLFLFAVWMV AFGVARQGI LRQNEQRWRW IFRSVIYEPY LAMFGQVPSD VDSTTYDFSH CTFSGNESKP LCVELDEHNL PRFPEWITIP L VCIYMLST NILLVNLLVA MFGYTVGIVQ ENNDQVWKFQ RYFLVQEYCN RLNIPFPFVV FAYFYMVVKK CFKCCCKEKN ME SNACCFR NEDNETLAWE GVMKENYLVK INTKANDNSE EMRHRFRQLD SKLNDLKSLL KEIANNIKSN SLEVLFQGPD YKD DDDKAH HHHHHHHHH UniProtKB: Transient receptor potential cation channel subfamily M member 8 |
-Macromolecule #2: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(o...
| Macromolecule | Name: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(oxidanyl)-4,5-diphosphonooxy-cyclohexyl]oxy-phosphoryl]oxy-propyl] octanoate type: ligand / ID: 2 / Number of copies: 4 / Formula: PIO |
|---|---|
| Molecular weight | Theoretical: 746.566 Da |
| Chemical component information |  ChemComp-PIO: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 12071 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8e4n: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)