+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of chi dynein bound to Lis1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | motor protein / dynein | |||||||||
| Function / homology |  Function and homology information Function and homology informationmicrotubule sliding / microtubule organizing center organization / nuclear migration along microtubule / microtubule plus-end binding / vesicle transport along microtubule / retrograde axonal transport / microtubule associated complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / cytoplasmic dynein complex ...microtubule sliding / microtubule organizing center organization / nuclear migration along microtubule / microtubule plus-end binding / vesicle transport along microtubule / retrograde axonal transport / microtubule associated complex / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / cytoplasmic dynein complex / nuclear migration / dynein intermediate chain binding / dynein complex binding / Antigen processing: Ubiquitination & Proteasome degradation / establishment of mitotic spindle orientation / cytoplasmic microtubule / cytoplasmic microtubule organization / mitotic spindle organization / kinetochore / spindle pole / nuclear envelope / cell cortex / cell division / ATP hydrolysis activity / ATP binding / identical protein binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Reimer JM / Lahiri I / Leschziner AE | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Lis1 relieves cytoplasmic dynein-1 autoinhibition by acting as a molecular wedge. Authors: Eva P Karasmanis / Janice M Reimer / Agnieszka A Kendrick / Kendrick H V Nguyen / Jennifer A Rodriguez / Joey B Truong / Indrajit Lahiri / Samara L Reck-Peterson / Andres E Leschziner /   Abstract: Cytoplasmic dynein-1 transports intracellular cargo towards microtubule minus ends. Dynein is autoinhibited and undergoes conformational changes to form an active complex that consists of one or two ...Cytoplasmic dynein-1 transports intracellular cargo towards microtubule minus ends. Dynein is autoinhibited and undergoes conformational changes to form an active complex that consists of one or two dynein dimers, the dynactin complex, and activating adapter(s). The Lissencephaly 1 gene, LIS1, is genetically linked to the dynein pathway from fungi to mammals and is mutated in people with the neurodevelopmental disease lissencephaly. Lis1 is required for active dynein complexes to form, but how it enables this is unclear. Here, we present a structure of two yeast dynein motor domains with two Lis1 dimers wedged in-between. The contact sites between dynein and Lis1 in this structure, termed 'Chi,' are required for Lis1's regulation of dynein in Saccharomyces cerevisiae in vivo and the formation of active human dynein-dynactin-activating adapter complexes in vitro. We propose that this structure represents an intermediate in dynein's activation pathway, revealing how Lis1 relieves dynein's autoinhibited state. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27810.map.gz emd_27810.map.gz | 157.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27810-v30.xml emd-27810-v30.xml emd-27810.xml emd-27810.xml | 18.6 KB 18.6 KB | Display Display |  EMDB header EMDB header |
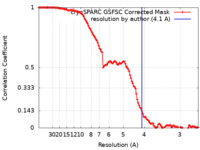
| FSC (resolution estimation) |  emd_27810_fsc.xml emd_27810_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_27810.png emd_27810.png | 57 KB | ||
| Others |  emd_27810_half_map_1.map.gz emd_27810_half_map_1.map.gz emd_27810_half_map_2.map.gz emd_27810_half_map_2.map.gz | 154 MB 154 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27810 http://ftp.pdbj.org/pub/emdb/structures/EMD-27810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27810 | HTTPS FTP |
-Related structure data
| Related structure data |  8dzzMC  8e00C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27810.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27810.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_27810_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
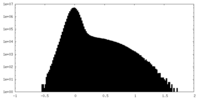
| Projections & Slices |
| ||||||||||||
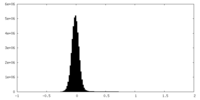
| Density Histograms |
-Half map: #1
| File | emd_27810_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
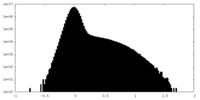
| Projections & Slices |
| ||||||||||||
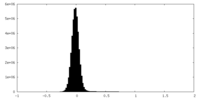
| Density Histograms |
- Sample components
Sample components
-Entire : Cytoplasmic dynein-1(E2448Q) bound to Lis1 in chi conformation
| Entire | Name: Cytoplasmic dynein-1(E2448Q) bound to Lis1 in chi conformation |
|---|---|
| Components |
|
-Supramolecule #1: Cytoplasmic dynein-1(E2448Q) bound to Lis1 in chi conformation
| Supramolecule | Name: Cytoplasmic dynein-1(E2448Q) bound to Lis1 in chi conformation type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Dynein heavy chain, cytoplasmic
| Macromolecule | Name: Dynein heavy chain, cytoplasmic / type: protein_or_peptide / ID: 1 / Details: 100% identical to UniParc UPI0005D9E17C / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 331.524 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GDQLTHVVEE VKTYDLVWRS IKNLWEDVQR TFETPWCRVD VLLLQSDLAN FLRRADELPR AVKQFEMYKS LFSQVNMLTS VNKILVELK DGALKPRHWN MIFRDIGKRQ IQKNLLDKLE FSLKDVMVLN LTLNEILLTK IIERAQKEFV IEKSLNRIKK F WKEAQYEV ...String: GDQLTHVVEE VKTYDLVWRS IKNLWEDVQR TFETPWCRVD VLLLQSDLAN FLRRADELPR AVKQFEMYKS LFSQVNMLTS VNKILVELK DGALKPRHWN MIFRDIGKRQ IQKNLLDKLE FSLKDVMVLN LTLNEILLTK IIERAQKEFV IEKSLNRIKK F WKEAQYEV IEHSSGLKLV REWDVLEQAC KEDLEELVSM KASNYYKIFE QDCLDLESKL TKLSEIQVNW VEVQFYWLDL YG ILGENLD IQNFLPLETS KFKSLTSEYK MITTRAFQLD TTIEVIHIPN FDTTLKLTID SLKMIKSSLS TFLERQRRQF PRF YFLGND DLLKIIGSGK HHDQVSKFMK KMFGSIESII FFEDSITGVR SVEGEVLNLN EKIELKDSIQ AQEWLNILDT EIKL SVFTQ FRDCLGQLKD GTDIEVVVSK YIFQAILLSA QVMWTELVEK CLQTNEFSKY WKEVDMKIKG LLDKLNKSSD NVKKK IEAL LVEYLHFNNV IGQLKNCSTK EEARLLWAKV QKFYQKNDTL DDLNSVFISQ SGYLLQYKFE YIGIPERLIY TPLLLV GFA TLTDSLHQKY GGCFFGPAGT GKTETVKAFG QNLGRVVVVF NCDDSFDYQV LSRLLVGITQ IGAWGCFDEF NRLDEKV LS AVSANIQQIQ NGLQVGKSHI TLLEEETPLS PHTAVFITLN PGYNGRSELP ENLKKSFREF SMKSPQSGTI AEMILQIM G FEDSKSLASK IVHFLELLSS KCSSMNHYHF GLRTLKGVLR NCSPLVSEFG EGEKTVVESL KRVILPSLGD TDELVFKDE LSKIFDSAGT PLNSKAIVQC LKDAGQRSGF SMSEEFLKKC MQFYYMQKTQ QALILVGKAG CGKTATWKTV IDAMAIFDGH ANVVYVIDT KVLTKESLYG SMLKATLEWR DGLFTSILRR VNDDITGTFK NSRIWVVFDS DLDPEYVEAM NSVLDDNKIL T LPNGERLP IPPNFRILFE TDNLDHTTPA TITRCGLLWF STDVCSISSK IDHLLNKSYE ALDNKLSMFE LDKLKDLISD SF DMASLTN IFTCSNDLVH ILGVRTFNKL ETAVQLAVHL ISSYRQWFQN LDDKSLKDVI TLLIKRSLLY ALAGDSTGES QRA FIQTIN TYFGHDSQEL SDYSTIVIAN DKLSFSSFCS EIPSVSLEAH EVMRPDIVIP TIDTIKHEKI FYDLLNSKRG IILC GPPGS GKTMIMNNAL RNSSLYDVVG INFSKDTTTE HILSALHRHT NYVTTSKGLT LLPKSDIKNL VLFCDQINLP KLDKY GSQN VVLFLRQLME KQGFWKTPEN KWVTIERIHI VGACNPPTDP GRIPMSERFT RHAAILYLGY PSGKSLSQIY EIYYKA IFK LVPEFRSYTE PFARASVHLY NECKARYSTG LQSHYLFSPR ELTRLVRGVY TAINTGPRQT LRSLIRLWAY EAWRIFA DR LVGVKEKNSF EQLLYETVDK YLPNQDLGNI SSTSLLFSGL LSLDFKEVNK TDLVNFIEER FKTFCDEELE VPMVIHES M VDHILRIDRA LKQVQGHMML IGASRTGKTI LTRFVAWLNG LKIVQPKIHR HSNLSDFDMI LKKAISDCSL KESRTCLII DESNILETAF LERMNTLLAN ADIPDLFQGE EYDKLLNNLR NKTRSLGLLL DTEQELYDWF VGEIAKNLHV VFTICDPTNN KSSAMISSP ALFNRCIINW MGDWDTKTMS QVANNMVDVV PMEFTDFIVP EVNKELVFTE PIQTIRDAVV NILIHFDRNF Y QKMKVGVN PRSPGYFIDG LRALVKLVTA KYQDLQENQR FVNVGLEKLN ESVLKVNELN KTLSKKSTEL TEKEKEARST LD KMLMEQN ESERKQEATE EIKKILKVQE EDIRKRKEVV MKSIQDIEPT ILEAQRGVKN IKKQQLTEIR SMVNPPSGVK IVM EAVCAI LGYQFSNWRD IQQFIRKDDF IHNIVHYDTT LHMKPQIRKY MEEEFLSDPN FTYETINRAS KACGPLYQWV NAQI NFSKV LENVDPLRQE MKRIEFESLK TKANLLAAEE MTQDLEASIE VSKQKYSLLI RDVEAIKTEM SNVQANLDRS ISLVK SLTF EKERWLNTTK QFSKTSQELI GNCIISSIYE TYFGHLNERE RGDMLVILKR LLGKFAVKYD VNYRFIDYLV TLDEKM KWL ECGLDKNDYF LENMSIVMNS QDAVPFLLDP SSHMITVISN YYGNKTVLLS FLEEGFVKRL ENAVRFGSVV IIQDGEF FD PIISRLISRE FNHAGNRVTV EIGDHEVDVS GDFKLFIHSC DPSGDIPIFL RSRVRLVHFV TNKESIETRI FDITLTEE N AEMQRKREDL IKLNTEYRLK LKNLEKRLLE ELNNSQGNML ENDELMVTLN NLKKEAMNIE KKLSESEEFF PQFDNLVEE YSIIGKHSVK IFSMLEKFGQ FHWFYGISIG QFLSCFKRVF IKKSRETRAA RTRVDEILWL LYQEVYCQFS TALDKKFKMI MAMTMFCLY KFDIESEQYK EAVLTMIGVL SESSDGVPKL TVDTNDDLRY LWDYVTTKSY ISALNWFKNE FFVDEWNIAD V VANSENNY FTMASERDVD GTFKLIELAK ASKESLKIIP LGSIENLNYA QEEISKSKIE GGWILLQNIQ MSLSWVKTYL HK HVEETKA AEEHEKFKMF MTCHLTGDKL PAPLLQRTDR VVYEDIPGIL DTVKDLWGSQ FFTGKISGVW SVYCTFLLSW FHA LITART RLVPHGFSKK YYFNDCDFQF ASVYLENVLA TNSTNNIPWA QVRDHIATIV YGGKIDEEKD LEVVAKLCAH VFCG SDNLQ IVPGVRIPQP LLQQSEEEER ARLTAILSNT IEPADSLSSW LQLPRESILD YERLQAKEVA SSTEQLLQEM UniProtKB: Dynein heavy chain, cytoplasmic |
-Macromolecule #2: Nuclear distribution protein PAC1
| Macromolecule | Name: Nuclear distribution protein PAC1 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.030617 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GMTNWQQQLP LTDTQKNELD KSVLRYLNWN YKQTVRHEHA QDYESVRHAI VTLSGFLLQE SVDRQEFISN NDTSNESMVD IDELLLPKK WNSIVRLQKK IIELEQNTET LVSQIKDLNT QVSELAQFKP TTSNGTSAHN VLKWIPRNLP SCLINVESSV T SVKLHPNL ...String: GMTNWQQQLP LTDTQKNELD KSVLRYLNWN YKQTVRHEHA QDYESVRHAI VTLSGFLLQE SVDRQEFISN NDTSNESMVD IDELLLPKK WNSIVRLQKK IIELEQNTET LVSQIKDLNT QVSELAQFKP TTSNGTSAHN VLKWIPRNLP SCLINVESSV T SVKLHPNL PIVFVATDHG KLYAFDLFNY TIPLASLQSH TKAITSMDVL FTNYTNSSKK NYLVIVTASK DLQIHVFKWV SE ECKFQQI RSLLGHEHIV SAVKIWQKNN DVHIASCSRD QTVKIWDFHN GWSLKTFQPH SQWVRSIDVL GDYIISGSHD TTL RLTHWP SGNGLSVGTG HEFPIEKVKF IHFIEDSPEI RFRTPSTDRY KNWGMQYCVS ASRDRTIKIW EIPLPTLMAH RAPI PNPTD SNFRCVLTLK GHLSWVRDIS IRGQYLFSCA DDKSVRCWDL NTGQCLHVWE KLHTGFVNCL DLDVDFDSNV TPRQM MVTG GLDCKSNVFM R UniProtKB: Nuclear distribution protein PAC1 |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 6 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 58.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.7 µm / Nominal defocus min: 2.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)