+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of LRRC8C-LRRC8A(IL125) Chimera, Class 4 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | LRRC8C / LRRC8A / SWELL / VRAC / Chimera / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpre-B cell differentiation / Miscellaneous transport and binding events / aspartate transmembrane transport / volume-sensitive anion channel activity / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / taurine transmembrane transport / monoatomic anion transmembrane transport / protein hexamerization / cellular response to osmotic stress ...pre-B cell differentiation / Miscellaneous transport and binding events / aspartate transmembrane transport / volume-sensitive anion channel activity / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / taurine transmembrane transport / monoatomic anion transmembrane transport / protein hexamerization / cellular response to osmotic stress / monoatomic anion transport / cell volume homeostasis / response to osmotic stress / intracellular glucose homeostasis / monoatomic ion channel complex / positive regulation of myoblast differentiation / fat cell differentiation / chloride transmembrane transport / positive regulation of insulin secretion / spermatogenesis / intracellular signal transduction / lysosomal membrane / endoplasmic reticulum membrane / cell surface / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
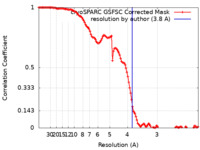
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Takahashi H / Yamada T / Denton JS / Strange K / Karakas E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: Cryo-EM structures of an LRRC8 chimera with native functional properties reveal heptameric assembly. Authors: Hirohide Takahashi / Toshiki Yamada / Jerod S Denton / Kevin Strange / Erkan Karakas /  Abstract: Volume-regulated anion channels (VRACs) mediate volume regulatory Cl and organic solute efflux from vertebrate cells. VRACs are heteromeric assemblies of LRRC8A-E proteins with unknown ...Volume-regulated anion channels (VRACs) mediate volume regulatory Cl and organic solute efflux from vertebrate cells. VRACs are heteromeric assemblies of LRRC8A-E proteins with unknown stoichiometries. Homomeric LRRC8A and LRRC8D channels have a small pore, hexameric structure. However, these channels are either non-functional or exhibit abnormal regulation and pharmacology, limiting their utility for structure-function analyses. We circumvented these limitations by developing novel homomeric LRRC8 chimeric channels with functional properties consistent with those of native VRAC/LRRC8 channels. We demonstrate here that the LRRC8C-LRRC8A(IL1) chimera comprising LRRC8C and 25 amino acids unique to the first intracellular loop (IL1) of LRRC8A has a heptameric structure like that of homologous pannexin channels. Unlike homomeric LRRC8A and LRRC8D channels, heptameric LRRC8C-LRRC8A(IL1) channels have a large-diameter pore similar to that estimated for native VRACs, exhibit normal DCPIB pharmacology, and have higher permeability to large organic anions. Lipid-like densities are located between LRRC8C-LRRC8A(IL1) subunits and occlude the channel pore. Our findings provide new insights into VRAC/LRRC8 channel structure and suggest that lipids may play important roles in channel gating and regulation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27773.map.gz emd_27773.map.gz | 168.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27773-v30.xml emd-27773-v30.xml emd-27773.xml emd-27773.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27773_fsc.xml emd_27773_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_27773.png emd_27773.png | 56.6 KB | ||
| Filedesc metadata |  emd-27773.cif.gz emd-27773.cif.gz | 6.3 KB | ||
| Others |  emd_27773_additional_1.map.gz emd_27773_additional_1.map.gz emd_27773_half_map_1.map.gz emd_27773_half_map_1.map.gz emd_27773_half_map_2.map.gz emd_27773_half_map_2.map.gz | 88.8 MB 165.1 MB 165.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27773 http://ftp.pdbj.org/pub/emdb/structures/EMD-27773 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27773 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27773 | HTTPS FTP |
-Related structure data
| Related structure data |  8dxqMC  8dxnC  8dxoC  8dxpC  8dxrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27773.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27773.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Unsharpened map.
| File | emd_27773_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_27773_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_27773_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : LRRC8C-LRRC8A(IL125) chimera
| Entire | Name: LRRC8C-LRRC8A(IL125) chimera |
|---|---|
| Components |
|
-Supramolecule #1: LRRC8C-LRRC8A(IL125) chimera
| Supramolecule | Name: LRRC8C-LRRC8A(IL125) chimera / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Heptameric LRRC8C-LRRC8A(IL125) chimera. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 700 KDa |
-Macromolecule #1: Volume-regulated anion channel subunit LRRC8C,Volume-regulated an...
| Macromolecule | Name: Volume-regulated anion channel subunit LRRC8C,Volume-regulated anion channel subunit LRRC8A type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 94.747695 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIPVTEFRQF SEQQPAFRVL KPWWDVFTDY LSVAMLMIGV FGCTLQVMQD KIICLPKRVQ PAQNHSSLSN VSQAVASTTP LPPPKPSPA NPITVEMKGL KTDLDLQQYS FINQMCYERA LHWYAKYFPY LVLIHTLVFM LCSNFWFKFP GSSSKIEHFI S ILGKCFDS ...String: MIPVTEFRQF SEQQPAFRVL KPWWDVFTDY LSVAMLMIGV FGCTLQVMQD KIICLPKRVQ PAQNHSSLSN VSQAVASTTP LPPPKPSPA NPITVEMKGL KTDLDLQQYS FINQMCYERA LHWYAKYFPY LVLIHTLVFM LCSNFWFKFP GSSSKIEHFI S ILGKCFDS PWTTRALSEV SGEDSDPKPA FSKMNGSMDK KSSTVSEDVE GSLVNSQSLK SIPEKFVVDK STAGALDKKE GE QAKALFE KVKKFRLHVE EGDILYAMYV RQTVLKVIKF LIIIAYNSAL VSKVQFTVDC NVDIQDMTGY KNFSCNHTMA HLF SKLSFC YLCFVSIYGL TCLYTLYWLF YRSLREYSFE YVRQETGIDD IPDVKNDFAF MLHMIDQYDP LYSKRFAVFL SEVS ENKLK QLNLNNEWTP DKLRQKLQTN AHNRLELPLI MLSGLPDTVF EITELQSLKL EIIKNVMIPA TIAQLDNLQE LSLHQ CSVK IHSAALSFLK ENLKVLSVKF DDMRELPPWM YGLRNLEELY LVGSLSHDIS RNVTLESLRD LKSLKILSIK SNVSKI PQA VVDVSSHLQK MCIHNDGTKL VMLNNLKKMT NLTELELVHC DLERIPHAVF SLLSLQELDL KENNLKSIEE IVSFQHL RK LTVLKLWHNS ITYIPEHIKK LTSLERLSFS HNKIEVLPSH LFLCNKIRYL DLSYNDIRFI PPEIGVLQSL QYFSITCN V ESLPDELYFC KKLKTLKIGK NSLSVLSPKI GNLLFLSYLD VKGNHFEILP PELGDCRALK RAGLVVEDAL FETLPSDVR EQMKTEENLY FQGAAAGDYK DDDDK UniProtKB: Volume-regulated anion channel subunit LRRC8C, Volume-regulated anion channel subunit LRRC8A, Volume-regulated anion channel subunit LRRC8C |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 3198 / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-8dxq: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)