[English] 日本語
 Yorodumi
Yorodumi- EMDB-27661: The 1.52 angstrom CryoEM structure of the [NiFe]-hydrogenase Huc ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The 1.52 angstrom CryoEM structure of the [NiFe]-hydrogenase Huc from Mycobacterium smegmatis - catalytic dimer (Huc2S2L) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | [NiFe] Hydrogenase / Membrane-associated / Complex / Quinone Transport / ELECTRON TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationhydrogenase (acceptor) / ferredoxin hydrogenase complex / [Ni-Fe] hydrogenase complex / hydrogenase (acceptor) activity / ferredoxin hydrogenase activity / cell envelope / anaerobic respiration / 3 iron, 4 sulfur cluster binding / nickel cation binding / 4 iron, 4 sulfur cluster binding ...hydrogenase (acceptor) / ferredoxin hydrogenase complex / [Ni-Fe] hydrogenase complex / hydrogenase (acceptor) activity / ferredoxin hydrogenase activity / cell envelope / anaerobic respiration / 3 iron, 4 sulfur cluster binding / nickel cation binding / 4 iron, 4 sulfur cluster binding / electron transfer activity / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) | |||||||||
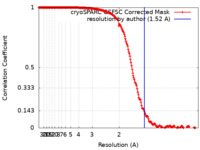
| Method | single particle reconstruction / cryo EM / Resolution: 1.52 Å | |||||||||
 Authors Authors | Grinter R / Venugopal H / Kropp A / Greening C | |||||||||
| Funding support |  Australia, 2 items Australia, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis for bacterial energy extraction from atmospheric hydrogen. Authors: Rhys Grinter / Ashleigh Kropp / Hari Venugopal / Moritz Senger / Jack Badley / Princess R Cabotaje / Ruyu Jia / Zehui Duan / Ping Huang / Sven T Stripp / Christopher K Barlow / Matthew ...Authors: Rhys Grinter / Ashleigh Kropp / Hari Venugopal / Moritz Senger / Jack Badley / Princess R Cabotaje / Ruyu Jia / Zehui Duan / Ping Huang / Sven T Stripp / Christopher K Barlow / Matthew Belousoff / Hannah S Shafaat / Gregory M Cook / Ralf B Schittenhelm / Kylie A Vincent / Syma Khalid / Gustav Berggren / Chris Greening /       Abstract: Diverse aerobic bacteria use atmospheric H as an energy source for growth and survival. This globally significant process regulates the composition of the atmosphere, enhances soil biodiversity and ...Diverse aerobic bacteria use atmospheric H as an energy source for growth and survival. This globally significant process regulates the composition of the atmosphere, enhances soil biodiversity and drives primary production in extreme environments. Atmospheric H oxidation is attributed to uncharacterized members of the [NiFe] hydrogenase superfamily. However, it remains unresolved how these enzymes overcome the extraordinary catalytic challenge of oxidizing picomolar levels of H amid ambient levels of the catalytic poison O and how the derived electrons are transferred to the respiratory chain. Here we determined the cryo-electron microscopy structure of the Mycobacterium smegmatis hydrogenase Huc and investigated its mechanism. Huc is a highly efficient oxygen-insensitive enzyme that couples oxidation of atmospheric H to the hydrogenation of the respiratory electron carrier menaquinone. Huc uses narrow hydrophobic gas channels to selectively bind atmospheric H at the expense of O, and 3 [3Fe-4S] clusters modulate the properties of the enzyme so that atmospheric H oxidation is energetically feasible. The Huc catalytic subunits form an octameric 833 kDa complex around a membrane-associated stalk, which transports and reduces menaquinone 94 Å from the membrane. These findings provide a mechanistic basis for the biogeochemically and ecologically important process of atmospheric H oxidation, uncover a mode of energy coupling dependent on long-range quinone transport, and pave the way for the development of catalysts that oxidize H in ambient air. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27661.map.gz emd_27661.map.gz | 683.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27661-v30.xml emd-27661-v30.xml emd-27661.xml emd-27661.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27661_fsc.xml emd_27661_fsc.xml | 18.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_27661.png emd_27661.png | 145 KB | ||
| Filedesc metadata |  emd-27661.cif.gz emd-27661.cif.gz | 6.6 KB | ||
| Others |  emd_27661_additional_1.map.gz emd_27661_additional_1.map.gz emd_27661_half_map_1.map.gz emd_27661_half_map_1.map.gz emd_27661_half_map_2.map.gz emd_27661_half_map_2.map.gz | 365.8 MB 677.1 MB 677.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27661 http://ftp.pdbj.org/pub/emdb/structures/EMD-27661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27661 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27661 | HTTPS FTP |
-Related structure data
| Related structure data |  8dqvMC  7utdC  7uurC  7uusC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27661.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27661.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.5 Å | ||||||||||||||||||||||||||||||||||||
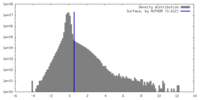
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_27661_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
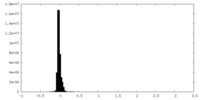
| Density Histograms |
-Half map: #2
| File | emd_27661_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
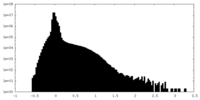
| Density Histograms |
-Half map: #1
| File | emd_27661_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
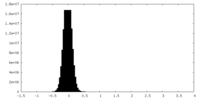
| Density Histograms |
- Sample components
Sample components
+Entire : Complex of the type 2 [NiFe]-hydrogenase Huc from Mycobacterium s...
+Supramolecule #1: Complex of the type 2 [NiFe]-hydrogenase Huc from Mycobacterium s...
+Macromolecule #1: Hydrogenase-2, large subunit
+Macromolecule #2: Hydrogenase-2, small subunit
+Macromolecule #3: OXYGEN ATOM
+Macromolecule #4: MAGNESIUM ION
+Macromolecule #5: CARBONMONOXIDE-(DICYANO) IRON
+Macromolecule #6: NICKEL (III) ION
+Macromolecule #7: MENADIONE
+Macromolecule #8: FE3-S4 CLUSTER
+Macromolecule #9: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.0 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.9 Component:
Details: pH 7.9 | |||||||||
| Grid | Model: Quantifoil / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 9868 / Average exposure time: 4.0 sec. / Average electron dose: 60.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: HELIUM |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: BACKBONE TRACE / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-8dqv: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)