+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Negative stain EM map of an MTA-HDAC-RBBP4 complex | |||||||||||||||||||||
 Map data Map data | Main map of MHR after homogeneous refinement. | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | Complex / Subcomplex / Deacetylase / Remodeller / Protein Binding / DNA BINDING PROTEIN | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
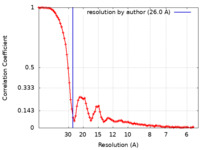
| Method | single particle reconstruction / negative staining / Resolution: 26.0 Å | |||||||||||||||||||||
 Authors Authors | Jackman MJ / Landsberg MJ / Viswanath S / Arvindekar S / Mackay JP | |||||||||||||||||||||
| Funding support |  Australia, Australia,  India, 6 items India, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Protein Sci / Year: 2022 Journal: Protein Sci / Year: 2022Title: Molecular architecture of nucleosome remodeling and deacetylase sub-complexes by integrative structure determination. Authors: Shreyas Arvindekar / Matthew J Jackman / Jason K K Low / Michael J Landsberg / Joel P Mackay / Shruthi Viswanath /   Abstract: The nucleosome remodeling and deacetylase (NuRD) complex is a chromatin-modifying assembly that regulates gene expression and DNA damage repair. Despite its importance, limited structural information ...The nucleosome remodeling and deacetylase (NuRD) complex is a chromatin-modifying assembly that regulates gene expression and DNA damage repair. Despite its importance, limited structural information describing the complete NuRD complex is available and a detailed understanding of its mechanism is therefore lacking. Drawing on information from SEC-MALLS, DIA-MS, XLMS, negative-stain EM, X-ray crystallography, NMR spectroscopy, secondary structure predictions, and homology models, we applied Bayesian integrative structure determination to investigate the molecular architecture of three NuRD sub-complexes: MTA1-HDAC1-RBBP4, MTA1 -HDAC1-MBD3 , and MTA1-HDAC1-RBBP4-MBD3-GATAD2A [nucleosome deacetylase (NuDe)]. The integrative structures were corroborated by examining independent crosslinks, cryo-EM maps, biochemical assays, known cancer-associated mutations, and structure predictions from AlphaFold. The robustness of the models was assessed by jack-knifing. Localization of the full-length MBD3, which connects the deacetylase and chromatin remodeling modules in NuRD, has not previously been possible; our models indicate two different locations for MBD3, suggesting a mechanism by which MBD3 in the presence of GATAD2A asymmetrically bridges the two modules in NuRD. Further, our models uncovered three previously unrecognized subunit interfaces in NuDe: HDAC1 -MTA1 , MTA1 -MBD3 , and HDAC1 -MBD3 . Our approach also allowed us to localize regions of unknown structure, such as HDAC1 and MBD3 , thereby resulting in the most complete and robustly cross-validated structural characterization of these NuRD sub-complexes so far. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27557.map.gz emd_27557.map.gz | 29.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27557-v30.xml emd-27557-v30.xml emd-27557.xml emd-27557.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27557_fsc.xml emd_27557_fsc.xml | 11.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_27557.png emd_27557.png | 19.4 KB | ||
| Masks |  emd_27557_msk_1.map emd_27557_msk_1.map | 59.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27557.cif.gz emd-27557.cif.gz | 5.6 KB | ||
| Others |  emd_27557_additional_1.map.gz emd_27557_additional_1.map.gz emd_27557_half_map_1.map.gz emd_27557_half_map_1.map.gz emd_27557_half_map_2.map.gz emd_27557_half_map_2.map.gz | 29 MB 55.2 MB 55.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27557 http://ftp.pdbj.org/pub/emdb/structures/EMD-27557 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27557 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27557 | HTTPS FTP |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27557.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27557.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map of MHR after homogeneous refinement. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.79 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27557_msk_1.map emd_27557_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Input used in the IMP for the generation...
| File | emd_27557_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Input used in the IMP for the generation of the predicted MHR model. It was derived from an independent processing run using the same dataset as the model used to generate the final map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B from homogeneous refinement that produced...
| File | emd_27557_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B from homogeneous refinement that produced final map of MHR. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A from homogeneous refinement that produced...
| File | emd_27557_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A from homogeneous refinement that produced final map of MHR. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Low resolution map of the MTA1:HDAC1:RBBP7 complex from HEK Expi2...
| Entire | Name: Low resolution map of the MTA1:HDAC1:RBBP7 complex from HEK Expi293F cells |
|---|---|
| Components |
|
-Supramolecule #1: Low resolution map of the MTA1:HDAC1:RBBP7 complex from HEK Expi2...
| Supramolecule | Name: Low resolution map of the MTA1:HDAC1:RBBP7 complex from HEK Expi293F cells type: complex / ID: 1 / Parent: 0 Details: Subcomplex that was generated via combination of recombinant proteins pulled down via FLAG tags from HEK Expi293F cells |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.015 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: This is the exchange buffer used for both concentrating the sample and also removing sucrose after GraFix. | ||||||||||||
| Staining | Type: NEGATIVE / Material: Uranyl Acetate Details: 5 uL of sample was applied to the grid and incubated for 2 minutes. The grid was then blotted and washed with 10 drops of distilled water, with liquid blotted off between each drop. The grid ...Details: 5 uL of sample was applied to the grid and incubated for 2 minutes. The grid was then blotted and washed with 10 drops of distilled water, with liquid blotted off between each drop. The grid was then pre-stained with a drop 1% Uranyl Acetate, blotted, then stained with a drop of 1% Uranyl Acetate for 30 seconds. It was then blotted again and left to air dry at room temperature. | ||||||||||||
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 5 / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Details | The sample was in high abundance, and appears to be well dispersed. Some aggregation is clear, and the particles upon closer inspection (such as 2D classification) are heterogeneous. This is likely due to the flexibility of the complex. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Image recording | Film or detector model: OTHER / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 469 / Average electron dose: 100.0 e/Å2 Details: Images recorded on a DE LC1100 lens coupled CCD detector. Estimated dose. |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 52000 |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)