[English] 日本語
 Yorodumi
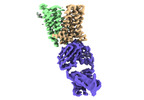
Yorodumi- EMDB-27497: Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc | |||||||||||||||
 Map data Map data | Cryo-EM map of human ferroportin/slc40 bound to Ca2 | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | SLC40 / Fpn / ferroportin / iron transporter / calcium / Ca2+ / human / nanodisc / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationspleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity / endothelium development / iron ion transmembrane transporter activity ...spleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity / endothelium development / iron ion transmembrane transporter activity / iron ion transmembrane transport / peptide hormone binding / establishment of localization in cell / Iron uptake and transport / multicellular organismal-level iron ion homeostasis / synaptic vesicle / basolateral plasma membrane / intracellular iron ion homeostasis / transcription by RNA polymerase II / apoptotic process / negative regulation of apoptotic process / positive regulation of transcription by RNA polymerase II / nucleoplasm / metal ion binding / identical protein binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||||||||
 Authors Authors | Shen J / Wilbon AS / Pan Y / Zhou M | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: Mechanism of Ca transport by ferroportin. Authors: Jiemin Shen / Azaan Saalim Wilbon / Ming Zhou / Yaping Pan /  Abstract: Ferroportin (Fpn) is a transporter that releases ferrous ion (Fe) from cells and is important for homeostasis of iron in circulation. Export of one Fe by Fpn is coupled to import of two H to maintain ...Ferroportin (Fpn) is a transporter that releases ferrous ion (Fe) from cells and is important for homeostasis of iron in circulation. Export of one Fe by Fpn is coupled to import of two H to maintain charge balance. Here, we show that human Fpn (HsFpn) binds to and mediates Ca transport. We determine the structure of Ca-bound HsFpn and identify a single Ca binding site distinct from the Fe binding sites. Further studies validate the Ca binding site and show that Ca transport is not coupled to transport of another ion. In addition, Ca transport is significantly inhibited in the presence of Fe but not vice versa. Function of Fpn as a Ca uniporter may allow regulation of iron homeostasis by Ca. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27497.map.gz emd_27497.map.gz | 15 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27497-v30.xml emd-27497-v30.xml emd-27497.xml emd-27497.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27497.png emd_27497.png | 55.6 KB | ||
| Filedesc metadata |  emd-27497.cif.gz emd-27497.cif.gz | 6.7 KB | ||
| Others |  emd_27497_half_map_1.map.gz emd_27497_half_map_1.map.gz emd_27497_half_map_2.map.gz emd_27497_half_map_2.map.gz | 28.2 MB 28.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27497 http://ftp.pdbj.org/pub/emdb/structures/EMD-27497 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27497 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27497 | HTTPS FTP |
-Related structure data
| Related structure data |  8dl6MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27497.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27497.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of human ferroportin/slc40 bound to Ca2 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half A map of human ferroportin/slc40 bound to Ca2
| File | emd_27497_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half_A map of human ferroportin/slc40 bound to Ca2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half B map of human ferroportin/slc40 bound to Ca2
| File | emd_27497_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half_B map of human ferroportin/slc40 bound to Ca2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc
| Entire | Name: Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc
| Supramolecule | Name: Cryo-EM structure of human ferroportin/slc40 bound to Ca2+ in nanodisc type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 40 member 1
| Macromolecule | Name: Solute carrier family 40 member 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 63.386621 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTRAGDHNRQ RGCCGSLADY LTSAKFLLYL GHSLSTWGDR MWHFAVSVFL VELYGNSLLL TAVYGLVVAG SVLVLGAIIG DWVDKNARL KVAQTSLVVQ NVSVILCGII LMMVFLHKHE LLTMYHGWVL TSCYILIITI ANIANLASTA TAITIQRDWI V VVAGEDRS ...String: MTRAGDHNRQ RGCCGSLADY LTSAKFLLYL GHSLSTWGDR MWHFAVSVFL VELYGNSLLL TAVYGLVVAG SVLVLGAIIG DWVDKNARL KVAQTSLVVQ NVSVILCGII LMMVFLHKHE LLTMYHGWVL TSCYILIITI ANIANLASTA TAITIQRDWI V VVAGEDRS KLANMNATIR RIDQLTNILA PMAVGQIMTF GSPVIGCGFI SGWNLVSMCV EYVLLWKVYQ KTPALAVKAG LK EEETELK QLNLHKDTEP KPLEGTHLMG VKDSNIHELE HEQEPTCASQ MAEPFRTFRD GWVSYYNQPV FLAGMGLAFL YMT VLGFDC ITTGYAYTQG LSGSILSILM GASAITGIMG TVAFTWLRRK CGLVRTGLIS GLAQLSCLIL CVISVFMPGS PLDL SVSPF EDIRSRFIQG ESITPTKIPE ITTEIYMSNG SNSANIVPET SPESVPIISV SLLFAGVIAA RIGLWSFDLT VTQLL QENV IESERGIING VQNSMNYLLD LLHFIMVILA PNPEAFGLLV LISVSFVAMG HIMYFRFAQN TLGNKLFACG PDAKEV RKE NQANTSVVEN LYFQ UniProtKB: Ferroportin |
-Macromolecule #2: 11F9 light-chain
| Macromolecule | Name: 11F9 light-chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.493885 KDa |
| Sequence | String: DIVMTQSQKF MSTSVGDRVS ITCKASQNVG TAVAWYQKKP GQSPKLLIYS ASNRYSGVPD RFTGSGSGTD FTLTISNMQS EDLADYFCQ QYGSYPLTFG SGTKLEIKEA EAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS ...String: DIVMTQSQKF MSTSVGDRVS ITCKASQNVG TAVAWYQKKP GQSPKLLIYS ASNRYSGVPD RFTGSGSGTD FTLTISNMQS EDLADYFCQ QYGSYPLTFG SGTKLEIKEA EAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS KDSTYSMSST LTLTKDEYER HNSYTCEATH KTSTSPIVKS FNRNE |
-Macromolecule #3: 11F9 heavy-chain
| Macromolecule | Name: 11F9 heavy-chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.815039 KDa |
| Sequence | String: MKCSWVIFFL MAVVTGVNSE VQLQQSGAEL VRPGALVKLS CKASGFNIKD YYMHWVKERP EQGLEWIGWI DPENGNTIYD PKFQGKASI TADTSSNTAY LQLSSLTSED TAVYYCARKR GYYGPYFDYW GQGTTLTVSS KTTAPSVYPL APVCGDTTGS S VTLGCLVK ...String: MKCSWVIFFL MAVVTGVNSE VQLQQSGAEL VRPGALVKLS CKASGFNIKD YYMHWVKERP EQGLEWIGWI DPENGNTIYD PKFQGKASI TADTSSNTAY LQLSSLTSED TAVYYCARKR GYYGPYFDYW GQGTTLTVSS KTTAPSVYPL APVCGDTTGS S VTLGCLVK GYFPEPVTLT WNSGSLSSGV HTFPAVLQSG LYTLSSSVTV TSSTWPSQSI TCNVAHPASS TKVDKKIEPA |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 1 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 305 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)