+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Citrus V-ATPase State 1, H in contact with subunit a | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | V-ATPase / rotary ATPase / complex / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationproton-transporting V-type ATPase, V1 domain / proton-transporting two-sector ATPase complex, catalytic domain / proton-transporting V-type ATPase, V0 domain / vacuolar proton-transporting V-type ATPase, V1 domain / vacuolar proton-transporting V-type ATPase, V0 domain / proton-transporting V-type ATPase complex / plant-type vacuole / actin filament capping / vacuolar acidification / vacuole ...proton-transporting V-type ATPase, V1 domain / proton-transporting two-sector ATPase complex, catalytic domain / proton-transporting V-type ATPase, V0 domain / vacuolar proton-transporting V-type ATPase, V1 domain / vacuolar proton-transporting V-type ATPase, V0 domain / proton-transporting V-type ATPase complex / plant-type vacuole / actin filament capping / vacuolar acidification / vacuole / vacuolar membrane / actin filament bundle assembly / proton-transporting ATPase activity, rotational mechanism / ATP metabolic process / H+-transporting two-sector ATPase / endomembrane system / transmembrane transport / actin filament binding / ATPase binding / ATP hydrolysis activity / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
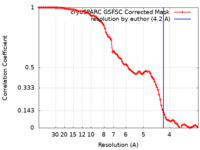
| Method | single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Keon KA / Abdelaziz RA / Schulze WX / Schumacher K / Rubinstein JL | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2022 Journal: Structure / Year: 2022Title: Structure of V-ATPase from citrus fruit. Authors: Yong Zi Tan / Kristine A Keon / Rana Abdelaziz / Peter Imming / Waltraud Schulze / Karin Schumacher / John L Rubinstein /   Abstract: We used the Legionella pneumophila effector SidK to affinity purify the endogenous vacuolar-type ATPases (V-ATPases) from lemon fruit. The preparation was sufficient for cryoelectron microscopy, ...We used the Legionella pneumophila effector SidK to affinity purify the endogenous vacuolar-type ATPases (V-ATPases) from lemon fruit. The preparation was sufficient for cryoelectron microscopy, allowing structure determination of the enzyme in two rotational states. The structure defines the ATP:H ratio of the enzyme, demonstrating that it can establish a maximum ΔpH of ∼3, which is insufficient to maintain the low pH observed in the vacuoles of juice sac cells in lemons and other citrus fruit. Compared with yeast and mammalian enzymes, the membrane region of the plant V-ATPase lacks subunit f and possesses an unusual configuration of transmembrane α helices. Subunit H, which inhibits ATP hydrolysis in the isolated catalytic region of V-ATPase, adopts two different conformations in the intact complex, hinting at a role in modulating activity in the intact enzyme. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26825.map.gz emd_26825.map.gz | 94.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26825-v30.xml emd-26825-v30.xml emd-26825.xml emd-26825.xml | 30.7 KB 30.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26825_fsc.xml emd_26825_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_26825.png emd_26825.png | 62.5 KB | ||
| Filedesc metadata |  emd-26825.cif.gz emd-26825.cif.gz | 8.2 KB | ||
| Others |  emd_26825_additional_1.map.gz emd_26825_additional_1.map.gz emd_26825_half_map_1.map.gz emd_26825_half_map_1.map.gz emd_26825_half_map_2.map.gz emd_26825_half_map_2.map.gz | 97.4 MB 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26825 http://ftp.pdbj.org/pub/emdb/structures/EMD-26825 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26825 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26825 | HTTPS FTP |
-Validation report
| Summary document |  emd_26825_validation.pdf.gz emd_26825_validation.pdf.gz | 900 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26825_full_validation.pdf.gz emd_26825_full_validation.pdf.gz | 899.6 KB | Display | |
| Data in XML |  emd_26825_validation.xml.gz emd_26825_validation.xml.gz | 18.2 KB | Display | |
| Data in CIF |  emd_26825_validation.cif.gz emd_26825_validation.cif.gz | 23.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26825 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26825 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26825 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26825 | HTTPS FTP |
-Related structure data
| Related structure data |  7uw9MC  7uwaC  7uwbC  7uwcC  7uwdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26825.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26825.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.617 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_26825_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_26825_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_26825_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Citrus V-ATPase
+Supramolecule #1: Citrus V-ATPase
+Macromolecule #1: V-type proton ATPase subunit H
+Macromolecule #2: V-type proton ATPase catalytic subunit A
+Macromolecule #3: V-type proton ATPase subunit B2
+Macromolecule #4: V-type proton ATPase subunit D
+Macromolecule #5: V-type proton ATPase subunit F
+Macromolecule #6: V-type proton ATPase subunit AP1 fragment
+Macromolecule #7: V-type proton ATPase subunit c"
+Macromolecule #8: V-type proton ATPase subunit d2
+Macromolecule #9: V-type proton ATPase subunit c
+Macromolecule #10: V-type proton ATPase subunit AP2 fragment
+Macromolecule #11: V-type proton ATPase subunit E
+Macromolecule #12: V-type proton ATPase subunit G
+Macromolecule #13: V-type proton ATPase subunit C
+Macromolecule #14: V-type proton ATPase subunit a3
+Macromolecule #15: V-type proton ATPase subunit e1
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 31.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.414211 µm / Nominal defocus min: 0.677558 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)