[English] 日本語
 Yorodumi
Yorodumi- EMDB-26141: Alpha1/BetaB Heteromeric Glycine Receptor in Glycine-Bound State -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Alpha1/BetaB Heteromeric Glycine Receptor in Glycine-Bound State | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Glycine / Ion Channel / Ligand-Gated / Receptor / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationNeurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / glycine binding / cellular response to zinc ion / cellular response to ethanol / ligand-gated monoatomic ion channel activity / response to amino acid ...Neurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / glycine binding / cellular response to zinc ion / cellular response to ethanol / ligand-gated monoatomic ion channel activity / response to amino acid / chloride channel complex / monoatomic ion transport / chloride transmembrane transport / central nervous system development / cellular response to amino acid stimulus / transmembrane signaling receptor activity / intracellular protein localization / perikaryon / postsynaptic membrane / dendrite / zinc ion binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
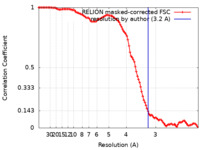
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Gibbs E / Chakrapani S / Kumar A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Conformational transitions and allosteric modulation in a heteromeric glycine receptor Authors: Gibbs E / Klemm E / Seiferth D / Kumar A / Ilca SL / Biggin PC / Chakrapani S | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26141.map.gz emd_26141.map.gz | 52.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26141-v30.xml emd-26141-v30.xml emd-26141.xml emd-26141.xml | 23.3 KB 23.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26141_fsc.xml emd_26141_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_26141.png emd_26141.png | 80.6 KB | ||
| Masks |  emd_26141_msk_1.map emd_26141_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-26141.cif.gz emd-26141.cif.gz | 7 KB | ||
| Others |  emd_26141_half_map_1.map.gz emd_26141_half_map_1.map.gz emd_26141_half_map_2.map.gz emd_26141_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26141 http://ftp.pdbj.org/pub/emdb/structures/EMD-26141 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26141 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26141 | HTTPS FTP |
-Related structure data
| Related structure data |  7tviMC  7tu9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26141.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26141.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
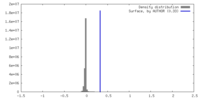
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26141_msk_1.map emd_26141_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
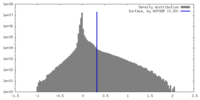
| Density Histograms |
-Half map: #2
| File | emd_26141_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
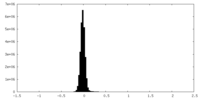
| Density Histograms |
-Half map: #1
| File | emd_26141_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
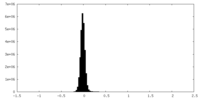
| Density Histograms |
- Sample components
Sample components
-Entire : Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor
| Entire | Name: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor |
|---|---|
| Components |
|
-Supramolecule #1: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor
| Supramolecule | Name: Zebrafish Alpha1 BetaB Heteromeric Glycine Receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: Glycine receptor subunit alphaZ1
| Macromolecule | Name: Glycine receptor subunit alphaZ1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 52.537598 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN ...String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN FPMDVQTCIM QLESFGYTMN DLIFEWDEKG AVQVADGLTL PQFILKEEKD LRYCTKHYNT GKFTCIEARF HL ERQMGYY LIQMYIPSLL IVILSWVSFW INMDAAPARV GLGITTVLTM TTQSSGSRAS LPKVSYVKAI DIWMAVCLLF VFS ALLEYA AVNFIARQHK ELLRFQRRRR HLKEDEAGDG RFSFAAYGMG PACLQAKDGM AIKGNNNNAP TSTNPPEKTV EEMR KLFIS RAKRIDTVSR VAFPLVFLIF NIFYWITYKI IRSEDIHKQL VPRGSHHHHH HHH UniProtKB: Glycine receptor subunit alphaZ1 |
-Macromolecule #2: Glycine receptor beta subunit 2
| Macromolecule | Name: Glycine receptor beta subunit 2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.820281 KDa |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MKALKVIFML LIICLWMEGG FTKEKSAKKW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGS WSHPQFEKEN LYFQGEKSAK KGKKKGKQVY CPSQLSSEDL ARVPANSTSN ILNKLLITYD PRIRPNFKGI P VEDRVNIF ...String: MKALKVIFML LIICLWMEGG FTKEKSAKKW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGSW SHPQFEKGGG SGGGSGGGS WSHPQFEKEN LYFQGEKSAK KGKKKGKQVY CPSQLSSEDL ARVPANSTSN ILNKLLITYD PRIRPNFKGI P VEDRVNIF INSFGSIQET TMDYRVNIFL RQRWNDPRLR LPQDFKSDSL TVDPKMFKCL WKPDLFFANE KSANFHDVTQ EN ILLFIFR NGDVLISMRL SVTLSCPLDL TLFPMDTQRC KMQLESFGYT TDDLQFMWQS GDPVQMDEIA LPQFDIKQED IEY GNCTKY YAGTGYYTCV EVIFTLRRQV GFYMMGVYAP TLLIVVLSWL SFWINPDASA ARVPLGILSV LSLSSECTSL ASEL PKVSY VKAIDIWLIA CLLFGFASLV EYAVVQVMLN SPKLLEAERA KIATKEKAEG KTPAKNTING MGSTPIHVST LQVTE TRCK KVCTSKSDLR TNDFSIVGSL PRDFELSNFD CYGKPIEVGS AFSKSQAKNN KKPPPPKPVI PSAAKRIDLY ARALFP FSF LFFNVIYWSV YLENLYFQGT ETSQVAPA UniProtKB: Glycine receptor subunit beta |
-Macromolecule #3: GLYCINE
| Macromolecule | Name: GLYCINE / type: ligand / ID: 3 / Number of copies: 5 / Formula: GLY |
|---|---|
| Molecular weight | Theoretical: 75.067 Da |
| Chemical component information |  ChemComp-GLY: |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 5 sec. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | Single Particle |
- Electron microscopy #1
Electron microscopy #1
| Microscopy ID | 1 |
|---|---|
| Microscope | FEI TITAN KRIOS |
| Image recording | Image recording ID: 1 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Electron microscopy #1~
Electron microscopy #1~
| Microscopy ID | 1 |
|---|---|
| Microscope | FEI TITAN KRIOS |
| Image recording | Image recording ID: 2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


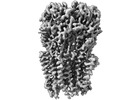



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)