+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Human potassium-chloride cotransporter 1 in inward-open state | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | SLC12A4 / Potassium-chloride transport / Inward-open state / TRANSPORT PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報potassium:chloride symporter activity / Cation-coupled Chloride cotransporters / chloride ion homeostasis / potassium ion homeostasis / cell volume homeostasis / potassium ion import across plasma membrane / monoatomic ion transport / potassium ion transmembrane transport / protein serine/threonine kinase binding / chloride transmembrane transport ...potassium:chloride symporter activity / Cation-coupled Chloride cotransporters / chloride ion homeostasis / potassium ion homeostasis / cell volume homeostasis / potassium ion import across plasma membrane / monoatomic ion transport / potassium ion transmembrane transport / protein serine/threonine kinase binding / chloride transmembrane transport / chemical synaptic transmission / lysosomal membrane / synapse / ATP binding / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.25 Å | |||||||||
 データ登録者 データ登録者 | Zhao YX / Cao EH | |||||||||
| 資金援助 | 1件
| |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2022 ジャーナル: Proc Natl Acad Sci U S A / 年: 2022タイトル: Structure of the human cation-chloride cotransport KCC1 in an outward-open state. 著者: Yongxiang Zhao / Jiemin Shen / Qinzhe Wang / Manuel Jose Ruiz Munevar / Pietro Vidossich / Marco De Vivo / Ming Zhou / Erhu Cao /   要旨: Cation-chloride cotransporters (CCCs) catalyze electroneutral symport of Cl with Na and/or K across membranes. CCCs are fundamental in cell volume homeostasis, transepithelia ion movement, ...Cation-chloride cotransporters (CCCs) catalyze electroneutral symport of Cl with Na and/or K across membranes. CCCs are fundamental in cell volume homeostasis, transepithelia ion movement, maintenance of intracellular Cl concentration, and neuronal excitability. Here, we present a cryoelectron microscopy structure of human K-Cl cotransporter (KCC)1 bound with the VU0463271 inhibitor in an outward-open state. In contrast to many other amino acid-polyamine-organocation transporter cousins, our first outward-open CCC structure reveals that opening the KCC1 extracellular ion permeation path does not involve hinge-bending motions of the transmembrane (TM) 1 and TM6 half-helices. Instead, rocking of TM3 and TM8, together with displacements of TM4, TM9, and a conserved intracellular loop 1 helix, underlie alternate opening and closing of extracellular and cytoplasmic vestibules. We show that KCC1 intriguingly exists in one of two distinct dimeric states via different intersubunit interfaces. Our studies provide a blueprint for understanding the mechanisms of CCCs and their inhibition by small molecule compounds. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_26115.map.gz emd_26115.map.gz | 59.7 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-26115-v30.xml emd-26115-v30.xml emd-26115.xml emd-26115.xml | 15.9 KB 15.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_26115.png emd_26115.png | 36.3 KB | ||
| Filedesc metadata |  emd-26115.cif.gz emd-26115.cif.gz | 6.5 KB | ||
| その他 |  emd_26115_additional_1.map.gz emd_26115_additional_1.map.gz | 59.1 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26115 http://ftp.pdbj.org/pub/emdb/structures/EMD-26115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26115 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_26115_validation.pdf.gz emd_26115_validation.pdf.gz | 512.9 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_26115_full_validation.pdf.gz emd_26115_full_validation.pdf.gz | 512.5 KB | 表示 | |
| XML形式データ |  emd_26115_validation.xml.gz emd_26115_validation.xml.gz | 6.3 KB | 表示 | |
| CIF形式データ |  emd_26115_validation.cif.gz emd_26115_validation.cif.gz | 7.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26115 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26115 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26115 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26115 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7tthMC  7ttiC C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_26115.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_26115.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.092 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: #1
| ファイル | emd_26115_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
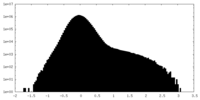
| 投影像・断面図 |
| ||||||||||||
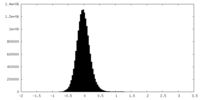
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Human Potassium-chloride cotransporter 1
| 全体 | 名称: Human Potassium-chloride cotransporter 1 |
|---|---|
| 要素 |
|
-超分子 #1: Human Potassium-chloride cotransporter 1
| 超分子 | 名称: Human Potassium-chloride cotransporter 1 / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Solute carrier family 12 member 4
| 分子 | 名称: Solute carrier family 12 member 4 / タイプ: protein_or_peptide / ID: 1 / コピー数: 2 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 120.770492 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MPHFTVVPVD GPRRGDYDNL EGLSWVDYGE RAELDDSDGH GNHRESSPFL SPLEASRGID YYDRNLALFE EELDIRPKVS SLLGKLVSY TNLTQGAKEH EEAESGEGTR RRAAEAPSMG TLMGVYLPCL QNIFGVILFL RLTWMVGTAG VLQALLIVLI C CCCTLLTA ...文字列: MPHFTVVPVD GPRRGDYDNL EGLSWVDYGE RAELDDSDGH GNHRESSPFL SPLEASRGID YYDRNLALFE EELDIRPKVS SLLGKLVSY TNLTQGAKEH EEAESGEGTR RRAAEAPSMG TLMGVYLPCL QNIFGVILFL RLTWMVGTAG VLQALLIVLI C CCCTLLTA ISMSAIATNG VVPAGGSYFM ISRSLGPEFG GAVGLCFYLG TTFAAAMYIL GAIEILLTYI APPAAIFYPS GA HDTSNAT LNNMRVYGTI FLTFMTLVVF VGVKYVNKFA SLFLACVIIS ILSIYAGGIK SIFDPPVFPV CMLGNRTLSR DQF DICAKT AVVDNETVAT QLWSFFCHSP NLTTDSCDPY FMLNNVTEIP GIPGAAAGVL QENLWSAYLE KGDIVEKHGL PSAD APSLK ESLPLYVVAD IATSFTVLVG IFFPSVTGIM AGSNRSGDLR DAQKSIPVGT ILAIITTSLV YFSSVVLFGA CIEGV VLRD KYGDGVSRNL VVGTLAWPSP WVIVIGSFFS TCGAGLQSLT GAPRLLQAIA KDNIIPFLRV FGHGKVNGEP TWALLL TAL IAELGILIAS LDMVAPILSM FFLMCYLFVN LACAVQTLLR TPNWRPRFKY YHWALSFLGM SLCLALMFVS SWYYALV AM LIAGMIYKYI EYQGAEKEWG DGIRGLSLSA ARYALLRLEE GPPHTKNWRP QLLVLLKLDE DLHVKYPRLL TFASQLKA G KGLTIVGSVI QGSFLESYGE AQAAEQTIKN MMEIEKVKGF CQVVVASKVR EGLAHLIQSC GLGGMRHNSV VLGWPYGWR QSEDPRAWKT FIDTVRCTTA AHLALLVPKN IAFYPSNHER YLEGHIDVWW IVHDGGMLML LPFLLRQHKV WRKCRMRIFT VAQMDDNSI QMKKDLAVFL YHLRLEAEVE VVEMHNSDIS AYTYERTLMM EQRSQMLRQM RLTKTERERE AQLVKDRHSA L RLESLYSD EEDESAVGAD KIQMTWTRDK YMTETWDPSH APDNFRELVH IKPDQSNVRR MHTAVKLNEV IVTRSHDARL VL LNMPGPP RNSEGDENYM EFLEVLTEGL ERVLLVRGGG REVITIYS UniProtKB: Solute carrier family 12 member 4 |
-分子 #3: POTASSIUM ION
| 分子 | 名称: POTASSIUM ION / タイプ: ligand / ID: 3 / コピー数: 2 / 式: K |
|---|---|
| 分子量 | 理論値: 39.098 Da |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 40.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.5 µm / 最小 デフォーカス(公称値): 1.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)