[English] 日本語
 Yorodumi
Yorodumi- EMDB-26113: BamABCDE bound to substrate EspP in the barrelized EspP/continuou... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BamABCDE bound to substrate EspP in the barrelized EspP/continuous open BamA state | |||||||||
 Map data Map data | BAM-MBP-76EspP barrelized EspP/continuous open BamA state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein folding / membrane dynamics / outer membrane protein / BAM / beta-barrel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationBam protein complex / Gram-negative-bacterium-type cell outer membrane assembly / detection of maltose stimulus / maltose transport complex / protein insertion into membrane / carbohydrate transport / Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases / carbohydrate transmembrane transporter activity / maltose binding / maltose transport ...Bam protein complex / Gram-negative-bacterium-type cell outer membrane assembly / detection of maltose stimulus / maltose transport complex / protein insertion into membrane / carbohydrate transport / Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases / carbohydrate transmembrane transporter activity / maltose binding / maltose transport / maltodextrin transmembrane transport / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / ATP-binding cassette (ABC) transporter complex / cell chemotaxis / cell outer membrane / outer membrane-bounded periplasmic space / periplasmic space / serine-type endopeptidase activity / DNA damage response / cell surface / proteolysis / extracellular region / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.8 Å | |||||||||
 Authors Authors | Doyle MT / Jimah JR | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Cryo-EM structures reveal multiple stages of bacterial outer membrane protein folding. Authors: Matthew Thomas Doyle / John R Jimah / Tyrone Dowdy / Shannon I Ohlemacher / Mioara Larion / Jenny E Hinshaw / Harris D Bernstein /  Abstract: Transmembrane β barrel proteins are folded into the outer membrane (OM) of Gram-negative bacteria by the β barrel assembly machinery (BAM) via a poorly understood process that occurs without known ...Transmembrane β barrel proteins are folded into the outer membrane (OM) of Gram-negative bacteria by the β barrel assembly machinery (BAM) via a poorly understood process that occurs without known external energy sources. Here, we used single-particle cryo-EM to visualize the folding dynamics of a model β barrel protein (EspP) by BAM. We found that BAM binds the highly conserved "β signal" motif of EspP to correctly orient β strands in the OM during folding. We also found that the folding of EspP proceeds via "hybrid-barrel" intermediates in which membrane integrated β sheets are attached to the essential BAM subunit, BamA. The structures show an unprecedented deflection of the membrane surrounding the EspP intermediates and suggest that β sheets progressively fold toward BamA to form a β barrel. Along with in vivo experiments that tracked β barrel folding while the OM tension was modified, our results support a model in which BAM harnesses OM elasticity to accelerate β barrel folding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26113.map.gz emd_26113.map.gz | 2.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26113-v30.xml emd-26113-v30.xml emd-26113.xml emd-26113.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26113_fsc.xml emd_26113_fsc.xml | 9 KB | Display |  FSC data file FSC data file |
| Images |  emd_26113.png emd_26113.png | 34.8 KB | ||
| Filedesc metadata |  emd-26113.cif.gz emd-26113.cif.gz | 13.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26113 http://ftp.pdbj.org/pub/emdb/structures/EMD-26113 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26113 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26113 | HTTPS FTP |
-Related structure data
| Related structure data |  7tt7MC  7tszC  7tt0C  7tt1C  7tt2C  7tt3C  7tt4C  7tt5C  7tt6C  7ttcC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26113.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26113.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BAM-MBP-76EspP barrelized EspP/continuous open BamA state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07382 Å | ||||||||||||||||||||||||||||||||||||
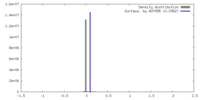
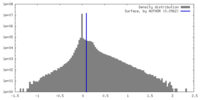
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : BamABCDE bound to substrate EspP in the barrelized EspP/continuou...
| Entire | Name: BamABCDE bound to substrate EspP in the barrelized EspP/continuous open BamA state |
|---|---|
| Components |
|
-Supramolecule #1: BamABCDE bound to substrate EspP in the barrelized EspP/continuou...
| Supramolecule | Name: BamABCDE bound to substrate EspP in the barrelized EspP/continuous open BamA state type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Outer membrane protein assembly factor BamA
| Macromolecule | Name: Outer membrane protein assembly factor BamA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 89.750094 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AHHHHHHHHG GEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVKE RPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP RNRVDLKLVF Q EGVSAEIQ ...String: AHHHHHHHHG GEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVKE RPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP RNRVDLKLVF Q EGVSAEIQ QINIVGNHAF TTDELISHFQ LRDEVPWWNV VGDRKYQKQK LAGDLETLRS YYLDRGYARF NIDSTQVSLT PD KKGIYVT VNITEGDQYK LSGVEVSGNL AGHSAEIEQL TKIEPGELYN GTKVTKMEDD IKKLLGRYGY AYPRVQSMPE IND ADKTVK LRVNVDAGNR FYVRKIRFEG NDTSKDAVLR REMRQMEGAW LGSDLVDQGK ERLNRLGFFE TVDTDTQRVP GSPD QVDVV YKVKERNTGC FNFGIGYGTE SGVSFQAGVQ QDNWLGTGYA VGINGTKNDY QTYAELSVTN PYFTVDGVSL GGRLF YNDF QADDADLSDY TNKSYGTDVT LGFPINEYNS LRAGLGYVHN SLSNMQPQVA MWRYLYSMGE HPSTSDQDNS FKTDDF TFN YGWTYNKLDR GYFPTDGSRV NLTGKVTIPG SDNEYYKVTL DTATYVPIDD DHKWVVLGRT RWGYGDGLGG KEMPFYE NF YAGGSSTVRG FQSNTIGPKA VYFPHQASNY DPDYDYECAT QDGAKDLCKS DDAVGGNAMA VASLEFITPT PFISDKYA N SVRTSFFWDM GTVWDTNWDS SQYSGYPDYS DPSNIRMSAG IALQWMSPLG PLVFSYAQPF KKYDGDKAEQ FQFNIGKTW UniProtKB: Outer membrane protein assembly factor BamA |
-Macromolecule #2: Outer membrane protein assembly factor BamC
| Macromolecule | Name: Outer membrane protein assembly factor BamC / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 34.40125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: CSSDSRYKRQ VSGDEAYLEA APLAELHAPA GMILPVTSGD YAIPVTNGSG AVGKALDIRP PAQPLALVSG ARTQFTGDTA SLLVENGRG NTLWPQVVSV LQAKNYTITQ RDDAGQTLTT DWVQWNRLDE DEQYRGRYQI SVKPQGYQQA VTVKLLNLEQ A GKPVADAA ...String: CSSDSRYKRQ VSGDEAYLEA APLAELHAPA GMILPVTSGD YAIPVTNGSG AVGKALDIRP PAQPLALVSG ARTQFTGDTA SLLVENGRG NTLWPQVVSV LQAKNYTITQ RDDAGQTLTT DWVQWNRLDE DEQYRGRYQI SVKPQGYQQA VTVKLLNLEQ A GKPVADAA SMQRYSTEMM NVISAGLDKS ATDAANAAQN RASTTMDVQS AADDTGLPML VVRGPFNVVW QRLPAALEKV GM KVTDSTR SQGNMAVTYK PLSDSDWQEL GASDPGLASG DYKLQVGDLD NRSSLQFIDP KGHTLTQSQN DALVAVFQAA FSK UniProtKB: Outer membrane protein assembly factor BamC |
-Macromolecule #3: Outer membrane protein assembly factor BamE
| Macromolecule | Name: Outer membrane protein assembly factor BamE / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.391554 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: CSTLERVVYR PDINQGNYLT ANDVSKIRVG MTQQQVAYAL GTPLMSDPFG TNTWFYVFRQ QPGHEGVTQQ TLTLTFNSSG VLTNIDNKP ALSGN UniProtKB: Outer membrane protein assembly factor BamE |
-Macromolecule #4: Outer membrane protein assembly factor BamD
| Macromolecule | Name: Outer membrane protein assembly factor BamD / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.816818 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: CSGSKEEVPD NPPNEIYATA QQKLQDGNWR QAITQLEALD NRYPFGPYSQ QVQLDLIYAY YKNADLPLAQ AAIDRFIRLN PTHPNIDYV MYMRGLTNMA LDDSALQGFF GVDRSDRDPQ HARAAFSDFS KLVRGYPNSQ YTTDATKRLV FLKDRLAKYE Y SVAEYYTE ...String: CSGSKEEVPD NPPNEIYATA QQKLQDGNWR QAITQLEALD NRYPFGPYSQ QVQLDLIYAY YKNADLPLAQ AAIDRFIRLN PTHPNIDYV MYMRGLTNMA LDDSALQGFF GVDRSDRDPQ HARAAFSDFS KLVRGYPNSQ YTTDATKRLV FLKDRLAKYE Y SVAEYYTE RGAWVAVVNR VEGMLRDYPD TQATRDALPL MENAYRQMQM NAQAEKVAKI IAANSSNT UniProtKB: Outer membrane protein assembly factor BamD |
-Macromolecule #5: Maltose/maltodextrin-binding periplasmic protein,Serine protease ...
| Macromolecule | Name: Maltose/maltodextrin-binding periplasmic protein,Serine protease EspP chimera type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 83.429984 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AWSHPQFEKG GGSGGGSGGS AWSHPQFEKG GAKIEEGKLV IWINGDKGYN GLAEVGKKFE KDTGIKVTVE HPDKLEEKFP QVAATGDGP DIIFWAHDRF GGYAQSGLLA EITPDKAFQD KLYPFTWDAV RYNGKLIAYP IAVEALSLIY NKDLLPNPPK T WEEIPALD ...String: AWSHPQFEKG GGSGGGSGGS AWSHPQFEKG GAKIEEGKLV IWINGDKGYN GLAEVGKKFE KDTGIKVTVE HPDKLEEKFP QVAATGDGP DIIFWAHDRF GGYAQSGLLA EITPDKAFQD KLYPFTWDAV RYNGKLIAYP IAVEALSLIY NKDLLPNPPK T WEEIPALD KELKAKGKSA LMFNLQEPYF TWPLIAADGG YAFKYENGKY DIKDVGVDNA GAKAGLTFLV DLIKNKHMNA DT DYSIAEA AFNKGETAMT INGPWAWSNI DTSKVNYGVT VLPTFKGQPS KPFVGVLSAG INAASPNKEL AKEFLENYLL TDE GLEAVN KDKPLGAVAL KSYEEELAKD PRIAATMENA QKGEIMPNIP QMSAFWYAVR TAVINAASGR QTVDEALKDA QTNS GSNIE LVSAPKDTNE NVFKASKQTI GFSDVTPVIT TRETGENLYF QGGDDKITWS LTGYNTVANK EATRNAAALF SVDYK AFLN EVNNLNKRMG DLRDINGEAG AWARIMSGTG SASGGFSDNY THVQVGVDKK HELDGLDLFT GFTVTHTDSS ASADVF SGK TKSVGAGLYA SAMFDSGAYI DLIGKYVHHD NEYTATFAGL GTRDYSTHSW YAGAEAGYRY HVTEDAWIEP QAELVYG SV SGKQFAWKDQ GMHLSMKDKD YNPLIGRTGV DVGKSFSGKD WKVTARAGLG YQFDLLANGE TVLRDASGEK RIKGEKDS R MLMSVGLNAE IRDNVRFGLE FEKSAFGKYN VDNAVNANFR YCF UniProtKB: Maltose/maltodextrin-binding periplasmic protein, Serine protease EspP |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | #0 - Image recording ID: 1 / #0 - Film or detector model: GATAN K2 SUMMIT (4k x 4k) / #0 - Average electron dose: 60.0 e/Å2 / #1 - Image recording ID: 2 / #1 - Film or detector model: GATAN K3 (6k x 4k) / #1 - Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.6 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)