[English] 日本語
 Yorodumi
Yorodumi- EMDB-25663: SARS-CoV-2 S (Spike Glycoprotein) D614G with Three (3) RBDs Up, B... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
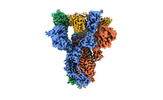
| Title | SARS-CoV-2 S (Spike Glycoprotein) D614G with Three (3) RBDs Up, Bound to Antibody 2-7 scFv, composite map | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | virus / coronavirus / SARS CoV-2 / SARS-CoV-2 / spike / 2-7 / D614G / RBD / 3 RBDs up / three RBDs up / scFv / complex / phage display / VIRAL PROTEIN / local refinement / focused refinement / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / receptor ligand activity / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
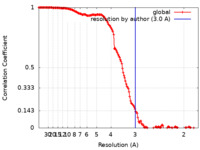
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Byrne PO / McLellan JS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: IgG-like bispecific antibodies with potent and synergistic neutralization against circulating SARS-CoV-2 variants of concern. Authors: Matthew R Chang / Luke Tomasovic / Natalia A Kuzmina / Adam J Ronk / Patrick O Byrne / Rebecca Johnson / Nadia Storm / Eduardo Olmedillas / Yixuan J Hou / Alexandra Schäfer / Sarah R Leist ...Authors: Matthew R Chang / Luke Tomasovic / Natalia A Kuzmina / Adam J Ronk / Patrick O Byrne / Rebecca Johnson / Nadia Storm / Eduardo Olmedillas / Yixuan J Hou / Alexandra Schäfer / Sarah R Leist / Longping V Tse / Hanzhong Ke / Christian Coherd / Katrina Nguyen / Maliwan Kamkaew / Anna Honko / Quan Zhu / Galit Alter / Erica Ollmann Saphire / Jason S McLellan / Anthony Griffiths / Ralph S Baric / Alexander Bukreyev / Wayne A Marasco /  Abstract: Monoclonal antibodies are a promising approach to treat COVID-19, however the emergence of SARS-CoV-2 variants has challenged the efficacy and future of these therapies. Antibody cocktails are being ...Monoclonal antibodies are a promising approach to treat COVID-19, however the emergence of SARS-CoV-2 variants has challenged the efficacy and future of these therapies. Antibody cocktails are being employed to mitigate these challenges, but neutralization escape remains a major challenge and alternative strategies are needed. Here we present two anti-SARS-CoV-2 spike binding antibodies, one Class 1 and one Class 4, selected from our non-immune human single-chain variable fragment (scFv) phage library, that are engineered into four, fully-human IgG-like bispecific antibodies (BsAb). Prophylaxis of hACE2 mice and post-infection treatment of golden hamsters demonstrates the efficacy of the monospecific antibodies against the original Wuhan strain, while promising in vitro results with the BsAbs demonstrate enhanced binding and distinct synergistic effects on neutralizing activity against circulating variants of concern. In particular, one BsAb engineered in a tandem scFv-Fc configuration shows synergistic neutralization activity against several variants of concern including B.1.617.2. This work provides evidence that synergistic neutralization can be achieved using a BsAb scaffold, and serves as a foundation for the future development of broadly reactive BsAbs against emerging variants of concern. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25663.map.gz emd_25663.map.gz | 295.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25663-v30.xml emd-25663-v30.xml emd-25663.xml emd-25663.xml | 43.3 KB 43.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25663_fsc.xml emd_25663_fsc.xml | 15.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_25663.png emd_25663.png | 53.7 KB | ||
| Masks |  emd_25663_msk_1.map emd_25663_msk_1.map emd_25663_msk_2.map emd_25663_msk_2.map emd_25663_msk_3.map emd_25663_msk_3.map | 325 MB 325 MB 325 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-25663.cif.gz emd-25663.cif.gz | 9.8 KB | ||
| Others |  emd_25663_additional_1.map.gz emd_25663_additional_1.map.gz emd_25663_additional_2.map.gz emd_25663_additional_2.map.gz emd_25663_additional_3.map.gz emd_25663_additional_3.map.gz emd_25663_additional_4.map.gz emd_25663_additional_4.map.gz emd_25663_additional_5.map.gz emd_25663_additional_5.map.gz emd_25663_half_map_1.map.gz emd_25663_half_map_1.map.gz emd_25663_half_map_2.map.gz emd_25663_half_map_2.map.gz | 160.2 MB 306.7 MB 160.2 MB 300.9 MB 300.9 MB 301.4 MB 301.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25663 http://ftp.pdbj.org/pub/emdb/structures/EMD-25663 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25663 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25663 | HTTPS FTP |
-Related structure data
| Related structure data |  7t3mMC  7t67C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25663.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25663.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||
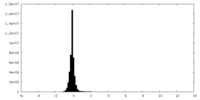
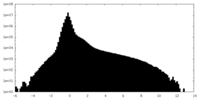
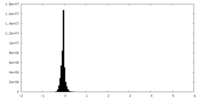
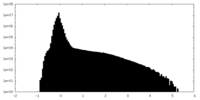
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Mask #1
+Mask #2
+Mask #3
+Additional map: #5
+Additional map: #4
+Additional map: #3
+Additional map: #2
+Additional map: #1
+Half map: #2
+Half map: #1
- Sample components
Sample components
-Entire : Complex of SARS CoV-2 S, Spike Glycoprotein, variant D614G with A...
| Entire | Name: Complex of SARS CoV-2 S, Spike Glycoprotein, variant D614G with Antibody 2-7 scFv |
|---|---|
| Components |
|
-Supramolecule #1: Complex of SARS CoV-2 S, Spike Glycoprotein, variant D614G with A...
| Supramolecule | Name: Complex of SARS CoV-2 S, Spike Glycoprotein, variant D614G with Antibody 2-7 scFv type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 127.00293 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF ...String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PINLVRDLPQ GFSALEPLVD LPIGINITRF QT LLALHRS YLTPGDSSSG WTAGAAAYYV GYLQPRTFLL KYNENGTITD AVDCALDPLS ETKCTLKSFT VEKGIYQTSN FRV QPTESI VRFPNITNLC PFGEVFNATR FASVYAWNRK RISNCVADYS VLYNSASFST FKCYGVSPTK LNDLCFTNVY ADSF VIRGD EVRQIAPGQT GKIADYNYKL PDDFTGCVIA WNSNNLDSKV GGNYNYLYRL FRKSNLKPFE RDISTEIYQA GSTPC NGVE GFNCYFPLQS YGFQPTNGVG YQPYRVVVLS FELLHAPATV CGPKKSTNLV KNKCVNFNFN GLTGTGVLTE SNKKFL PFQ QFGRDIADTT DAVRDPQTLE ILDITPCSFG GVSVITPGTN TSNQVAVLYQ GVNCTEVPVA IHADQLTPTW RVYSTGS NV FQTRAGCLIG AEHVNNSYEC DIPIGAGICA SYQTQTNSPG SASSVASQSI IAYTMSLGAE NSVAYSNNSI AIPTNFTI S VTTEILPVSM TKTSVDCTMY ICGDSTECSN LLLQYGSFCT QLNRALTGIA VEQDKNTQEV FAQVKQIYKT PPIKDFGGF NFSQILPDPS KPSKRSFIED LLFNKVTLAD AGFIKQYGDC LGDIAARDLI CAQKFNGLTV LPPLLTDEMI AQYTSALLAG TITSGWTFG AGAALQIPFA MQMAYRFNGI GVTQNVLYEN QKLIANQFNS AIGKIQDSLS STASALGKLQ DVVNQNAQAL N TLVKQLSS NFGAISSVLN DILSRLDPPE AEVQIDRLIT GRLQSLQTYV TQQLIRAAEI RASANLAATK MSECVLGQSK RV DFCGKGY HLMSFPQSAP HGVVFLHVTY VPAQEKNFTT APAICHDGKA HFPREGVFVS NGTHWFVTQR NFYEPQIITT DNT FVSGNC DVVIGIVNNT VYDPLQPELD SFK UniProtKB: Spike glycoprotein |
-Macromolecule #2: Antibody 2-7 scFv
| Macromolecule | Name: Antibody 2-7 scFv / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.570977 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKHLWFFLLL VAAAQPAMAQ VTLKESGPTR VKPTQTLTLT CTFSGFSLST TGVGVGWIRQ PPGKALEWLA LIYWNDDKRY SPSLKSRLT ITKDTSKNQV VLTMTNMDPV DTATYYCARI SGSGYFYPFD IWGQGTTVTV SSGGGGSGGG GSGGGGSNFM L TQPHSVSE ...String: MKHLWFFLLL VAAAQPAMAQ VTLKESGPTR VKPTQTLTLT CTFSGFSLST TGVGVGWIRQ PPGKALEWLA LIYWNDDKRY SPSLKSRLT ITKDTSKNQV VLTMTNMDPV DTATYYCARI SGSGYFYPFD IWGQGTTVTV SSGGGGSGGG GSGGGGSNFM L TQPHSVSE SPGKTVTISC TRSSGSIASN YVQWYQQRPG SSPTTVIYED NQRPSGVPDR FSGSIDSSSN SASLTISGLK AE DEADYYC QSYDSSSLWV FGGGTKLTVL GQPKAAPSAA ALEHHHHHH |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 45 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 180 sec. / Pretreatment - Atmosphere: OTHER | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)