[English] 日本語
 Yorodumi
Yorodumi- EMDB-25650: Activated state of 2-APB and CBD-bound wildtype rat TRPV2 in nanodiscs -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Activated state of 2-APB and CBD-bound wildtype rat TRPV2 in nanodiscs | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRP channel / ion channel / TRPV / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationgrowth cone membrane / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion import across plasma membrane / positive regulation of axon extension / axonal growth cone / monoatomic cation channel activity / endomembrane system / calcium channel activity ...growth cone membrane / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion import across plasma membrane / positive regulation of axon extension / axonal growth cone / monoatomic cation channel activity / endomembrane system / calcium channel activity / melanosome / lamellipodium / positive regulation of cold-induced thermogenesis / cell body / axon / negative regulation of cell population proliferation / cell surface / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Pumroy RA / Protopopova AD | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural insights into TRPV2 activation by small molecules. Authors: Ruth A Pumroy / Anna D Protopopova / Tabea C Fricke / Iris U Lange / Ferdinand M Haug / Phuong T Nguyen / Pamela N Gallo / Bárbara B Sousa / Gonçalo J L Bernardes / Vladimir Yarov-Yarovoy ...Authors: Ruth A Pumroy / Anna D Protopopova / Tabea C Fricke / Iris U Lange / Ferdinand M Haug / Phuong T Nguyen / Pamela N Gallo / Bárbara B Sousa / Gonçalo J L Bernardes / Vladimir Yarov-Yarovoy / Andreas Leffler / Vera Y Moiseenkova-Bell /     Abstract: Transient receptor potential vanilloid 2 (TRPV2) is involved in many critical physiological and pathophysiological processes, making it a promising drug target. Here we present cryo-electron ...Transient receptor potential vanilloid 2 (TRPV2) is involved in many critical physiological and pathophysiological processes, making it a promising drug target. Here we present cryo-electron microscopy (cryo-EM) structures of rat TRPV2 in lipid nanodiscs activated by 2-aminoethoxydiphenyl borate (2-APB) and propose a TRPV2-specific 2-ABP binding site at the interface of S5 of one monomer and the S4-S5 linker of the adjacent monomer. In silico docking and electrophysiological studies confirm the key role of His521 and Arg539 in 2-APB activation of TRPV2. Additionally, electrophysiological experiments show that the combination of 2-APB and cannabidiol has a synergetic effect on TRPV2 activation, and cryo-EM structures demonstrate that both drugs were able to bind simultaneously. Together, our cryo-EM structures represent multiple functional states of the channel, providing a native picture of TRPV2 activation by small molecules and a structural framework for the development of TRPV2-specific activators. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25650.map.gz emd_25650.map.gz | 59.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25650-v30.xml emd-25650-v30.xml emd-25650.xml emd-25650.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25650.png emd_25650.png | 124.3 KB | ||
| Filedesc metadata |  emd-25650.cif.gz emd-25650.cif.gz | 5.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25650 http://ftp.pdbj.org/pub/emdb/structures/EMD-25650 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25650 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25650 | HTTPS FTP |
-Related structure data
| Related structure data |  7t37MC  7n0mC  7n0nC  7t38C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25650.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25650.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Tetramer of wildtype rat TRPV2
| Entire | Name: Tetramer of wildtype rat TRPV2 |
|---|---|
| Components |
|
-Supramolecule #1: Tetramer of wildtype rat TRPV2
| Supramolecule | Name: Tetramer of wildtype rat TRPV2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 2
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 2 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 86.798891 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTSASSPPAF RLETSDGDEE GNAEVNKGKQ EPPPMESPFQ REDRNSSPQI KVNLNFIKRP PKNTSAPSQQ EPDRFDRDRL FSVVSRGVP EELTGLLEYL RWNSKYLTDS AYTEGSTGKT CLMKAVLNLQ DGVNACIMPL LQIDKDSGNP KLLVNAQCTD E FYQGHSAL ...String: MTSASSPPAF RLETSDGDEE GNAEVNKGKQ EPPPMESPFQ REDRNSSPQI KVNLNFIKRP PKNTSAPSQQ EPDRFDRDRL FSVVSRGVP EELTGLLEYL RWNSKYLTDS AYTEGSTGKT CLMKAVLNLQ DGVNACIMPL LQIDKDSGNP KLLVNAQCTD E FYQGHSAL HIAIEKRSLQ CVKLLVENGA DVHLRACGRF FQKHQGTCFY FGELPLSLAA CTKQWDVVTY LLENPHQPAS LE ATDSLGN TVLHALVMIA DNSPENSALV IHMYDGLLQM GARLCPTVQL EEISNHQGLT PLKLAAKEGK IEIFRHILQR EFS GPYQPL SRKFTEWCYG PVRVSLYDLS SVDSWEKNSV LEIIAFHCKS PNRHRMVVLE PLNKLLQEKW DRLVSRFFFN FACY LVYMF IFTVVAYHQP SLDQPAIPSS KATFGESMLL LGHILILLGG IYLLLGQLWY FWRRRLFIWI SFMDSYFEIL FLLQA LLTV LSQVLRFMET EWYLPLLVLS LVLGWLNLLY YTRGFQHTGI YSVMIQKVIL RDLLRFLLVY LVFLFGFAVA LVSLSR EAR SPKAPEDNNS TVTEQPTVGQ EEEPAPYRSI LDASLELFKF TIGMGELAFQ EQLRFRGVVL LLLLAYVLLT YVLLLNM LI ALMSETVNHV ADNSWSIWKL QKAISVLEME NGYWWCRRKK HREGRLLKVG TRGDGTPDER WCFRVEEVNW AAWEKTLP T LSEDPSGPGI TGNKKNPTSK PGKNSASEED HLPLQVLQSP UniProtKB: Transient receptor potential cation channel subfamily V member 2 |
-Macromolecule #2: cannabidiol
| Macromolecule | Name: cannabidiol / type: ligand / ID: 2 / Number of copies: 4 / Formula: P0T |
|---|---|
| Molecular weight | Theoretical: 314.462 Da |
| Chemical component information |  ChemComp-P0T: |
-Macromolecule #3: 2-aminoethyl diphenylborinate
| Macromolecule | Name: 2-aminoethyl diphenylborinate / type: ligand / ID: 3 / Number of copies: 4 / Formula: FZ4 |
|---|---|
| Molecular weight | Theoretical: 225.094 Da |
| Chemical component information | 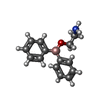 ChemComp-FZ4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 42.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 30563 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















