[English] 日本語
 Yorodumi
Yorodumi- EMDB-25633: Structure of electron bifurcating Ni-Fe hydrogenase complex HydAB... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25633 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of electron bifurcating Ni-Fe hydrogenase complex HydABCSL in FMN-free apo state | |||||||||
 Map data Map data | Electron bifurcating Ni-Fe hydrogenase complex HydABCSL in FMN-free apo state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | hydrogenase complex / electron bifurcation / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationferredoxin hydrogenase activity / nickel cation binding / NADH dehydrogenase activity / iron-sulfur cluster binding / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / 2 iron, 2 sulfur cluster binding / FMN binding / 4 iron, 4 sulfur cluster binding ...ferredoxin hydrogenase activity / nickel cation binding / NADH dehydrogenase activity / iron-sulfur cluster binding / NADH dehydrogenase (ubiquinone) activity / quinone binding / ATP synthesis coupled electron transport / 2 iron, 2 sulfur cluster binding / FMN binding / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Acetomicrobium mobile (bacteria) Acetomicrobium mobile (bacteria) | |||||||||
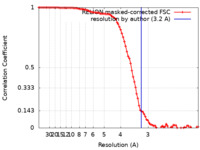
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Feng X / Li H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Structure and electron transfer pathways of an electron-bifurcating NiFe-hydrogenase. Authors: Xiang Feng / Gerrit J Schut / Dominik K Haja / Michael W W Adams / Huilin Li /  Abstract: Electron bifurcation enables thermodynamically unfavorable biochemical reactions. Four groups of bifurcating flavoenzyme are known and three use FAD to bifurcate. FeFe-HydABC hydrogenase represents ...Electron bifurcation enables thermodynamically unfavorable biochemical reactions. Four groups of bifurcating flavoenzyme are known and three use FAD to bifurcate. FeFe-HydABC hydrogenase represents the fourth group, but its bifurcation site is unknown. We report cryo-EM structures of the related NiFe-HydABCSL hydrogenase that reversibly oxidizes H and couples endergonic reduction of ferredoxin with exergonic reduction of NAD. FMN surrounded by a unique arrangement of iron sulfur clusters forms the bifurcating center. NAD binds to FMN in HydB, and electrons from H via HydA to a HydB [4Fe-4S] cluster enable the FMN to reduce NAD. Low-potential electron transfer from FMN to the HydC [2Fe-2S] cluster and subsequent reduction of a uniquely penta-coordinated HydB [2Fe-2S] cluster require conformational changes, leading to ferredoxin binding and reduction by a [4Fe-4S] cluster in HydB. This work clarifies the electron transfer pathways for a large group of hydrogenases underlying many essential functions in anaerobic microorganisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25633.map.gz emd_25633.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25633-v30.xml emd-25633-v30.xml emd-25633.xml emd-25633.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25633_fsc.xml emd_25633_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_25633.png emd_25633.png | 83.5 KB | ||
| Filedesc metadata |  emd-25633.cif.gz emd-25633.cif.gz | 6.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25633 http://ftp.pdbj.org/pub/emdb/structures/EMD-25633 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25633 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25633 | HTTPS FTP |
-Related structure data
| Related structure data |  7t2rMC  7t30C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25633.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25633.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Electron bifurcating Ni-Fe hydrogenase complex HydABCSL in FMN-free apo state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.029 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : NiFe hydrogenase complex ABCSL
+Supramolecule #1: NiFe hydrogenase complex ABCSL
+Macromolecule #1: NiFe hydrogenase subunit A
+Macromolecule #2: NiFe hydrogenase subunit B
+Macromolecule #3: NiFe hydrogenase subunit C
+Macromolecule #4: NiFe hydrogenase large subunit
+Macromolecule #5: NiFe hydrogenase small subunit
+Macromolecule #6: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #7: IRON/SULFUR CLUSTER
+Macromolecule #8: NICKEL (III) ION
+Macromolecule #9: CARBONMONOXIDE-(DICYANO) IRON
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 | |||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 76.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: -2.0 µm / Nominal defocus min: -1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7t2r: |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)