+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | T-Plastin-F-actin complex, parallel bundled state | |||||||||
 Map data Map data | main map, consensus | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationactin filament network formation / Striated Muscle Contraction / actin filament bundle / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / stress fiber / skeletal muscle fiber development / actin filament / bone development ...actin filament network formation / Striated Muscle Contraction / actin filament bundle / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / stress fiber / skeletal muscle fiber development / actin filament / bone development / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / actin filament binding / actin cytoskeleton / hydrolase activity / calcium ion binding / ATP binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.0 Å | |||||||||
 Authors Authors | Mei L / Reynolds MJ / Alushin GM | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Structural mechanism for bidirectional actin cross-linking by T-plastin. Authors: Lin Mei / Matthew J Reynolds / Damien Garbett / Rui Gong / Tobias Meyer / Gregory M Alushin /  Abstract: To orchestrate cell mechanics, trafficking, and motility, cytoskeletal filaments must assemble into higher-order networks whose local subcellular architecture and composition specify their functions. ...To orchestrate cell mechanics, trafficking, and motility, cytoskeletal filaments must assemble into higher-order networks whose local subcellular architecture and composition specify their functions. Cross-linking proteins bridge filaments at the nanoscale to control a network's μm-scale geometry, thereby conferring its mechanical properties and functional dynamics. While these interfilament linkages are key determinants of cytoskeletal function, their structural mechanisms remain poorly understood. Plastins/fimbrins are an evolutionarily ancient family of tandem calponin-homology domain (CHD) proteins required to construct multiple classes of actin networks, which feature diverse geometries specialized to power cytokinesis, microvilli and stereocilia biogenesis, and persistent cell migration. Here, we focus on the structural basis of actin network assembly by human T-plastin, a ubiquitously expressed isoform necessary for the maintenance of stable cellular protrusions generated by actin polymerization forces. By implementing a machine-learning-enabled cryo-electron microscopy pipeline for visualizing cross-linkers bridging multiple filaments, we uncover a sequential bundling mechanism enabling T-plastin to bridge pairs of actin filaments in both parallel and antiparallel orientations. T-plastin populates distinct structural landscapes in these two bridging orientations that are selectively compatible with actin networks featuring divergent architectures and functions. Our structural, biochemical, and cell biological data highlight inter-CHD linkers as key structural elements underlying flexible but stable cross-linking that are likely to be disrupted by T-plastin mutations that cause hereditary bone diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25494.map.gz emd_25494.map.gz | 57.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25494-v30.xml emd-25494-v30.xml emd-25494.xml emd-25494.xml | 32 KB 32 KB | Display Display |  EMDB header EMDB header |
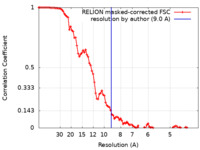
| FSC (resolution estimation) |  emd_25494_fsc.xml emd_25494_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_25494.png emd_25494.png | 35.7 KB | ||
| Masks |  emd_25494_msk_1.map emd_25494_msk_1.map emd_25494_msk_2.map emd_25494_msk_2.map emd_25494_msk_3.map emd_25494_msk_3.map | 64 MB 64 MB 64 MB |  Mask map Mask map | |
| Others |  emd_25494_additional_1.map.gz emd_25494_additional_1.map.gz emd_25494_additional_2.map.gz emd_25494_additional_2.map.gz emd_25494_additional_3.map.gz emd_25494_additional_3.map.gz emd_25494_additional_4.map.gz emd_25494_additional_4.map.gz emd_25494_additional_5.map.gz emd_25494_additional_5.map.gz emd_25494_additional_6.map.gz emd_25494_additional_6.map.gz emd_25494_half_map_1.map.gz emd_25494_half_map_1.map.gz emd_25494_half_map_2.map.gz emd_25494_half_map_2.map.gz | 58.5 MB 59 MB 44.2 MB 44.3 MB 44.4 MB 44.3 MB 49.5 MB 49.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25494 http://ftp.pdbj.org/pub/emdb/structures/EMD-25494 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25494 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25494 | HTTPS FTP |
-Related structure data
| Related structure data |  7sx8MC  7r94C  7sx9C  7sxaC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25494.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25494.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map, consensus | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Mask #1
+Mask #2
+Mask #3
+Additional map: main map, body 1
+Additional map: main map, body 2
+Additional map: half-map 1, body002
+Additional map: half-map 1, body001
+Additional map: half-map 2, body001
+Additional map: half-map 2, body002
+Half map: half-map 2, consensus
+Half map: half-map 1, consensus
- Sample components
Sample components
-Entire : T-Plastin-F-actin complex, parallel bundled state
| Entire | Name: T-Plastin-F-actin complex, parallel bundled state |
|---|---|
| Components |
|
-Supramolecule #1: T-Plastin-F-actin complex, parallel bundled state
| Supramolecule | Name: T-Plastin-F-actin complex, parallel bundled state / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Parallel actin bundles formed by actin-crosslinking T-plastin |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 27.5 kDa/nm |
-Macromolecule #1: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.109973 KDa |
| Sequence | String: MCDEDETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY ...String: MCDEDETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY EGYALPHAIM RLDLAGRDLT DYLMKILTER GYSFVTTAER EIVRDIKEKL CYVALDFENE MATAASSS S LEKSYELPDG QVITIGNERF RCPETLFQPS FIGMESAGIH ETTYNSIMKC DIDIRKDLYA NNVMSGGTTM YPGIADRMQ KEITALAPST MKIKIIAPPE RKYSVWIGGS ILASLSTFQQ MWITKQEYDE AGPSIVHRKC F |
-Macromolecule #2: Plastin-3
| Macromolecule | Name: Plastin-3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 70.896867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDEMATTQIS KDELDELKEA FAKVDLNSNG FICDYELHEL FKEANMPLPG YKVREIIQKL MLDGDRNKDG KISFDEFVYI FQEVKSSDI AKTFRKAINR KEGICALGGT SELSSEGTQH SYSEEEKYAF VNWINKALEN DPDCRHVIPM NPNTDDLFKA V GDGIVLCK ...String: MDEMATTQIS KDELDELKEA FAKVDLNSNG FICDYELHEL FKEANMPLPG YKVREIIQKL MLDGDRNKDG KISFDEFVYI FQEVKSSDI AKTFRKAINR KEGICALGGT SELSSEGTQH SYSEEEKYAF VNWINKALEN DPDCRHVIPM NPNTDDLFKA V GDGIVLCK MINLSVPDTI DERAINKKKL TPFIIQENLN LALNSASAIG CHVVNIGAED LRAGKPHLVL GLLWQIIKIG LF ADIELSR NEALAALLRD GETLEELMKL SPEELLLRWA NFHLENSGWQ KINNFSADIK DSKAYFHLLN QIAPKGQKEG EPR IDINMS GFNETDDLKR AESMLQQADK LGCRQFVTPA DVVSGNPKLN LAFVANLFNK YPALTKPENQ DIDWTLLEGE TREE RTFRN WMNSLGVNPH VNHLYADLQD ALVILQLYER IKVPVDWSKV NKPPYPKLGA NMKKLENCNY AVELGKHPAK FSLVG IGGQ DLNDGNQTLT LALVWQLMRR YTLNVLEDLG DGQKANDDII VNWVNRTLSE AGKSTSIQSF KDKTISSSLA VVDLID AIQ PGCINYDLVK SGNLTEDDKH NNAKYAVSMA RRIGARVYAL PEDLVEVKPK MVMTVFACLM GRGMKRV |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 6 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 6 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Average exposure time: 10.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)