[English] 日本語
 Yorodumi
Yorodumi- EMDB-25377: Cryo-EM structure of mouse agonist ML-SA1-bound TRPML1 channel at... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25377 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mouse agonist ML-SA1-bound TRPML1 channel at 2.32 Angstrom resolution | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationTransferrin endocytosis and recycling / positive regulation of lysosome organization / calcium ion export / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / NAADP-sensitive calcium-release channel activity / phagosome maturation / iron ion transmembrane transporter activity / iron ion transmembrane transport / cellular response to pH / TRP channels ...Transferrin endocytosis and recycling / positive regulation of lysosome organization / calcium ion export / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / NAADP-sensitive calcium-release channel activity / phagosome maturation / iron ion transmembrane transporter activity / iron ion transmembrane transport / cellular response to pH / TRP channels / monoatomic anion channel activity / endosomal transport / intracellular vesicle / sodium channel activity / monoatomic cation transmembrane transport / autophagosome maturation / potassium channel activity / monoatomic cation channel activity / phagocytic cup / release of sequestered calcium ion into cytosol / cellular response to calcium ion / cell projection / calcium channel activity / phagocytic vesicle membrane / late endosome / late endosome membrane / protein homotetramerization / adaptive immune response / lysosome / receptor complex / lysosomal membrane / lipid binding / Golgi apparatus / nucleoplasm / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.32 Å | |||||||||
 Authors Authors | Gan N / Han Y | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Structural mechanism of allosteric activation of TRPML1 by PI(3,5)P and rapamycin. Authors: Ninghai Gan / Yan Han / Weizhong Zeng / Yan Wang / Jing Xue / Youxing Jiang /  Abstract: Transient receptor potential mucolipin 1 (TRPML1) is a Ca-permeable, nonselective cation channel ubiquitously expressed in the endolysosomes of mammalian cells and its loss-of-function mutations are ...Transient receptor potential mucolipin 1 (TRPML1) is a Ca-permeable, nonselective cation channel ubiquitously expressed in the endolysosomes of mammalian cells and its loss-of-function mutations are the direct cause of type IV mucolipidosis (MLIV), an autosomal recessive lysosomal storage disease. TRPML1 is a ligand-gated channel that can be activated by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P] as well as some synthetic small-molecule agonists. Recently, rapamycin has also been shown to directly bind and activate TRPML1. Interestingly, both PI(3,5)P and rapamycin have low efficacy in channel activation individually but together they work cooperatively and activate the channel with high potency. To reveal the structural basis underlying the synergistic activation of TRPML1 by PI(3,5)P and rapamycin, we determined the high-resolution cryoelectron microscopy (cryo-EM) structures of the mouse TRPML1 channel in various states, including apo closed, PI(3,5)P-bound closed, and PI(3,5)P/temsirolimus (a rapamycin analog)-bound open states. These structures, combined with electrophysiology, elucidate the molecular details of ligand binding and provide structural insight into how the TRPML1 channel integrates two distantly bound ligand stimuli and facilitates channel opening. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25377.map.gz emd_25377.map.gz | 84.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25377-v30.xml emd-25377-v30.xml emd-25377.xml emd-25377.xml | 10.5 KB 10.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25377.png emd_25377.png | 50 KB | ||
| Filedesc metadata |  emd-25377.cif.gz emd-25377.cif.gz | 5.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25377 http://ftp.pdbj.org/pub/emdb/structures/EMD-25377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25377 | HTTPS FTP |
-Related structure data
| Related structure data |  7sq6MC  7sq7C  7sq8C  7sq9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_25377.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25377.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Mouse Transient receptor potential-mucolipin 1 apo closed state
| Entire | Name: Mouse Transient receptor potential-mucolipin 1 apo closed state |
|---|---|
| Components |
|
-Supramolecule #1: Mouse Transient receptor potential-mucolipin 1 apo closed state
| Supramolecule | Name: Mouse Transient receptor potential-mucolipin 1 apo closed state type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Mucolipin-1
| Macromolecule | Name: Mucolipin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.573617 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATPAGRRAS ETERLLTPNP GYGTQVGTSP APTTPTEEED LRRRLKYFFM SPCDKFRAKG RKPCKLMLQV VKILVVTVQL ILFGLSNQL VVTFREENTI AFRHLFLLGY SDGSDDTFAA YTQEQLYQAI FYAVDQYLIL PEISLGRYAY VRGGGGPWAN G SALALCQR ...String: MATPAGRRAS ETERLLTPNP GYGTQVGTSP APTTPTEEED LRRRLKYFFM SPCDKFRAKG RKPCKLMLQV VKILVVTVQL ILFGLSNQL VVTFREENTI AFRHLFLLGY SDGSDDTFAA YTQEQLYQAI FYAVDQYLIL PEISLGRYAY VRGGGGPWAN G SALALCQR YYHRGHVDPA NDTFDIDPRV VTDCIQVDPP DRPPDIPSED LDFLDGSASY KNLTLKFHKL INVTIHFQLK TI NLQSLIN NEIPDCYTFS ILITFDNKAH SGRIPIRLET KTHIQECKHP SVSRHGDNSF RLLFDVVVIL TCSLSFLLCA RSL LRGFLL QNEFVVFMWR RRGREISLWE RLEFVNGWYI LLVTSDVLTI SGTVMKIGIE AKNLASYDVC SILLGTSTLL VWVG VIRYL TFFHKYNILI ATLRVALPSV MRFCCCVAVI YLGYCFCGWI VLGPYHVKFR SLSMVSECLF SLINGDDMFV TFAAM QAQQ GHSSLVWLFS QLYLYSFISL FIYMVLSLFI ALITGAYDTI KHPGGTGTEK SELQAYIEQC QDSPTSGKFR RGSGSA CSL FCCCGRDSPE DHSLLVN UniProtKB: Mucolipin-1 |
-Macromolecule #3: 2-{2-oxo-2-[(4S)-2,2,4-trimethyl-3,4-dihydroquinolin-1(2H)-yl]eth...
| Macromolecule | Name: 2-{2-oxo-2-[(4S)-2,2,4-trimethyl-3,4-dihydroquinolin-1(2H)-yl]ethyl}-1H-isoindole-1,3(2H)-dione type: ligand / ID: 3 / Number of copies: 4 / Formula: AQV |
|---|---|
| Molecular weight | Theoretical: 362.422 Da |
| Chemical component information | 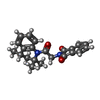 ChemComp-AQV: |
-Macromolecule #4: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 4 / Number of copies: 2 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 12 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.9 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.32 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 41980 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: RANDOM ASSIGNMENT |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















