+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Nucleotide-free Get3 in the closed form | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Tail-anchored membrane protein targeting Deviant Walker A ATPase targeting factor / CHAPERONE | |||||||||
| 生物種 |  Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし) Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし) | |||||||||
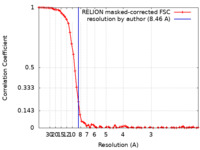
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 8.46 Å | |||||||||
 データ登録者 データ登録者 | Fry MY / Clemons Jr WM | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Nat Struct Mol Biol / 年: 2022 ジャーナル: Nat Struct Mol Biol / 年: 2022タイトル: Structurally derived universal mechanism for the catalytic cycle of the tail-anchored targeting factor Get3. 著者: Michelle Y Fry / Vladimíra Najdrová / Ailiena O Maggiolo / Shyam M Saladi / Pavel Doležal / William M Clemons /   要旨: Tail-anchored (TA) membrane proteins, accounting for roughly 2% of proteomes, are primarily targeted posttranslationally to the endoplasmic reticulum membrane by the guided entry of TA proteins (GET) ...Tail-anchored (TA) membrane proteins, accounting for roughly 2% of proteomes, are primarily targeted posttranslationally to the endoplasmic reticulum membrane by the guided entry of TA proteins (GET) pathway. For this complicated process, it remains unknown how the central targeting factor Get3 uses nucleotide to facilitate large conformational changes to recognize then bind clients while also preventing exposure of hydrophobic surfaces. Here, we identify the GET pathway in Giardia intestinalis and present the structure of the Get3-client complex in the critical postnucleotide-hydrolysis state, demonstrating that Get3 reorganizes the client-binding domain (CBD) to accommodate and shield the client transmembrane helix. Four additional structures of GiGet3, spanning the nucleotide-free (apo) open to closed transition and the ATP-bound state, reveal the details of nucleotide stabilization and occluded CBD. This work resolves key conundrums and allows for a complete model of the dramatic conformational landscape of Get3. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_25375.map.gz emd_25375.map.gz | 25.2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-25375-v30.xml emd-25375-v30.xml emd-25375.xml emd-25375.xml | 14.4 KB 14.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_25375_fsc.xml emd_25375_fsc.xml | 7 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_25375.png emd_25375.png | 48.8 KB | ||
| マスクデータ |  emd_25375_msk_1.map emd_25375_msk_1.map | 27 MB |  マスクマップ マスクマップ | |
| Filedesc metadata |  emd-25375.cif.gz emd-25375.cif.gz | 4.9 KB | ||
| その他 |  emd_25375_half_map_1.map.gz emd_25375_half_map_1.map.gz emd_25375_half_map_2.map.gz emd_25375_half_map_2.map.gz | 20.7 MB 20.7 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25375 http://ftp.pdbj.org/pub/emdb/structures/EMD-25375 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25375 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25375 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_25375_validation.pdf.gz emd_25375_validation.pdf.gz | 919.1 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_25375_full_validation.pdf.gz emd_25375_full_validation.pdf.gz | 918.7 KB | 表示 | |
| XML形式データ |  emd_25375_validation.xml.gz emd_25375_validation.xml.gz | 12.3 KB | 表示 | |
| CIF形式データ |  emd_25375_validation.cif.gz emd_25375_validation.cif.gz | 17 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25375 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25375 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25375 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25375 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_25375.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_25375.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.059 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-マスク #1
| ファイル |  emd_25375_msk_1.map emd_25375_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_25375_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_25375_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Nucleotide-free Get3 in a closed conformation
| 全体 | 名称: Nucleotide-free Get3 in a closed conformation |
|---|---|
| 要素 |
|
-超分子 #1: Nucleotide-free Get3 in a closed conformation
| 超分子 | 名称: Nucleotide-free Get3 in a closed conformation / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし) Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし)株: ATCC 50803 / WB clone C6 |
| 分子量 | 理論値: 78 KDa |
-分子 #1: ATPase GET3
| 分子 | 名称: ATPase GET3 / タイプ: protein_or_peptide / ID: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし) Giardia intestinalis (strain ATCC 50803 / WB clone C6) (やつひげはらむし) |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MLPSLHDILD QHTYKWIFFG GKGGVGKTTT SSSFSVLMAE TRPNEKFLLL STDPAHNISD AFDQKFGKAP TQVSGIPNLY AMEVDASNEM KSAVEAVQKE TGSAADNDAE SKSEGDMFGG LNDLITCASS FIKDGTFPGM DEMWSFINLI KLIDTNEYST VIFDTAPTGH ...文字列: MLPSLHDILD QHTYKWIFFG GKGGVGKTTT SSSFSVLMAE TRPNEKFLLL STDPAHNISD AFDQKFGKAP TQVSGIPNLY AMEVDASNEM KSAVEAVQKE TGSAADNDAE SKSEGDMFGG LNDLITCASS FIKDGTFPGM DEMWSFINLI KLIDTNEYST VIFDTAPTGH TLRFLELPET VNKVLEIFTR LKDNMGGMLS MVMQTMGLSQ NDIFGLIDKT YPKIDVVKRI SAEFRDPSLC TFVGVCIPEF LSLYETERLV QRLAVLDMDC HAIVINFVLD ANAATPCSMC RSRARMQNKY IDQINELYDD FNIVLSPLRH DEVRGIANLR DYAETLIKPY RFCWSANPDP SSAK |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 0.55 mg/mL |
|---|---|
| 緩衝液 | pH: 7.5 |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | エネルギーフィルター - 名称: GIF Bioquantum / エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 実像数: 9300 / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: SPOT SCAN / 撮影モード: DARK FIELD / 倍率(公称値): 88000 |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)