[English] 日本語
 Yorodumi
Yorodumi- EMDB-25170: Human wildtype GABA reuptake transporter 1 in complex with tiagab... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human wildtype GABA reuptake transporter 1 in complex with tiagabine, inward-open conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Neurotransmitter transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationgamma-aminobutyric acid reuptake / Reuptake of GABA / inorganic anion import across plasma membrane / sodium:chloride symporter activity / gamma-aminobutyric acid transmembrane transporter activity / gamma-aminobutyric acid import / gamma-aminobutyric acid:sodium:chloride symporter activity / sodium ion import across plasma membrane / SLC-mediated transport of neurotransmitters / amino acid transport ...gamma-aminobutyric acid reuptake / Reuptake of GABA / inorganic anion import across plasma membrane / sodium:chloride symporter activity / gamma-aminobutyric acid transmembrane transporter activity / gamma-aminobutyric acid import / gamma-aminobutyric acid:sodium:chloride symporter activity / sodium ion import across plasma membrane / SLC-mediated transport of neurotransmitters / amino acid transport / associative learning / transport across blood-brain barrier / sodium ion transmembrane transport / chloride transmembrane transport / synapse organization / GABA-ergic synapse / memory / presynapse / chemical synaptic transmission / axon / neuronal cell body / cell surface / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.82 Å | |||||||||
 Authors Authors | Gati C / Motiwala Z | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structural basis of GABA reuptake inhibition. Authors: Zenia Motiwala / Nanda Gowtham Aduri / Hamidreza Shaye / Gye Won Han / Jordy Homing Lam / Vsevolod Katritch / Vadim Cherezov / Cornelius Gati /  Abstract: γ-Aminobutyric acid (GABA) transporter 1 (GAT1) regulates neuronal excitation of the central nervous system by clearing the synaptic cleft of the inhibitory neurotransmitter GABA upon its release ...γ-Aminobutyric acid (GABA) transporter 1 (GAT1) regulates neuronal excitation of the central nervous system by clearing the synaptic cleft of the inhibitory neurotransmitter GABA upon its release from synaptic vesicles. Elevating the levels of GABA in the synaptic cleft, by inhibiting GABA reuptake transporters, is an established strategy to treat neurological disorders, such as epilepsy. Here we determined the cryo-electron microscopy structure of full-length, wild-type human GAT1 in complex with its clinically used inhibitor tiagabine, with an ordered part of only 60 kDa. Our structure reveals that tiagabine locks GAT1 in the inward-open conformation, by blocking the intracellular gate of the GABA release pathway, and thus suppresses neurotransmitter uptake. Our results provide insights into the mixed-type inhibition of GAT1 by tiagabine, which is an important anticonvulsant medication. Its pharmacodynamic profile, confirmed by our experimental data, suggests initial binding of tiagabine to the substrate-binding site in the outward-open conformation, whereas our structure presents the drug stalling the transporter in the inward-open conformation, consistent with a two-step mechanism of inhibition. The presented structure of GAT1 gives crucial insights into the biology and pharmacology of this important neurotransmitter transporter and provides blueprints for the rational design of neuromodulators, as well as moving the boundaries of what is considered possible in single-particle cryo-electron microscopy of challenging membrane proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25170.map.gz emd_25170.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25170-v30.xml emd-25170-v30.xml emd-25170.xml emd-25170.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25170.png emd_25170.png | 49.2 KB | ||
| Filedesc metadata |  emd-25170.cif.gz emd-25170.cif.gz | 6.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25170 http://ftp.pdbj.org/pub/emdb/structures/EMD-25170 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25170 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25170 | HTTPS FTP |
-Related structure data
| Related structure data |  7sk2MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25170.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25170.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.813 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : GAT-1 in complex with tiagabine
| Entire | Name: GAT-1 in complex with tiagabine |
|---|---|
| Components |
|
-Supramolecule #1: GAT-1 in complex with tiagabine
| Supramolecule | Name: GAT-1 in complex with tiagabine / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 67 kDa/nm |
-Macromolecule #1: Sodium- and chloride-dependent GABA transporter 1
| Macromolecule | Name: Sodium- and chloride-dependent GABA transporter 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 64.886793 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MATNGSKVAD GQISTEVSEA PVANDKPKTL VVKVQKKAAD LPDRDTWKGR FDFLMSCVGY AIGLGNVWRF PYLCGKNGGG AFLIPYFLT LIFAGVPLFL LECSLGQYTS IGGLGVWKLA PMFKGVGLAA AVLSFWLNIY YIVIISWAIY YLYNSFTTTL P WKQCDNPW ...String: MATNGSKVAD GQISTEVSEA PVANDKPKTL VVKVQKKAAD LPDRDTWKGR FDFLMSCVGY AIGLGNVWRF PYLCGKNGGG AFLIPYFLT LIFAGVPLFL LECSLGQYTS IGGLGVWKLA PMFKGVGLAA AVLSFWLNIY YIVIISWAIY YLYNSFTTTL P WKQCDNPW NTDRCFSNYS MVNTTNMTSA VVEFWERNMH QMTDGLDKPG QIRWPLAITL AIAWILVYFC IWKGVGWTGK VV YFSATYP YIMLIILFFR GVTLPGAKEG ILFYITPNFR KLSDSEVWLD AATQIFFSYG LGLGSLIALG SYNSFHNNVY RDS IIVCCI NSCTSMFAGF VIFSIVGFMA HVTKRSIADV AASGPGLAFL AYPEAVTQLP ISPLWAILFF SMLLMLGIDS QFCT VEGFI TALVDEYPRL LRNRRELFIA AVCIISYLIG LSNITQGGIY VFKLFDYYSA SGMSLLFLVF FECVSISWFY GVNRF YDNI QEMVGSRPCI WWKLCWSFFT PIIVAGVFIF SAVQMTPLTM GNYVFPKWGQ GVGWLMALSS MVLIPGYMAY MFLTLK GSL KQRIQVMVQP SEDIV UniProtKB: Sodium- and chloride-dependent GABA transporter 1 |
-Macromolecule #2: Tiagabine
| Macromolecule | Name: Tiagabine / type: ligand / ID: 2 / Number of copies: 1 / Formula: TGI |
|---|---|
| Molecular weight | Theoretical: 375.548 Da |
| Chemical component information | 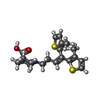 ChemComp-TGI: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 18 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 4000.0 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 39572 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 25.0 µm / Nominal defocus min: 10.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















