[English] 日本語
 Yorodumi
Yorodumi- EMDB-24791: Head region of a complex of IGF-I with the ectodomain of a hybrid... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Head region of a complex of IGF-I with the ectodomain of a hybrid insulin receptor / type 1 insulin-like growth factor receptor | |||||||||
 Map data Map data | Head region of a complex of IGF-I with the ectodomain of a hybrid insulin receptor / type 1 insulin-like growth factor receptor | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | insulin receptor / type 1 insulin like growth factor receptor / hybrid receptor / insulin like growth factor I / leucine zipper / cryo electron microscopy / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationglycolate metabolic process / muscle hypertrophy / negative regulation of oocyte development / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / positive regulation of trophectodermal cell proliferation / prostate gland stromal morphogenesis / positive regulation of glycoprotein biosynthetic process / positive regulation of type B pancreatic cell proliferation / type II pneumocyte differentiation ...glycolate metabolic process / muscle hypertrophy / negative regulation of oocyte development / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / positive regulation of trophectodermal cell proliferation / prostate gland stromal morphogenesis / positive regulation of glycoprotein biosynthetic process / positive regulation of type B pancreatic cell proliferation / type II pneumocyte differentiation / neuronal dense core vesicle lumen / proteoglycan biosynthetic process / regulation of establishment or maintenance of cell polarity / chondroitin sulfate proteoglycan biosynthetic process / protein kinase complex / myotube cell development / positive regulation of transcription regulatory region DNA binding / insulin-like growth factor receptor activity / negative regulation of neuroinflammatory response / insulin-like growth factor binding / Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / bone mineralization involved in bone maturation / protein transporter activity / positive regulation of cell growth involved in cardiac muscle cell development / IRS-related events triggered by IGF1R / negative regulation of vascular associated smooth muscle cell apoptotic process / positive regulation of cerebellar granule cell precursor proliferation / exocytic vesicle / lung vasculature development / regulation of female gonad development / transcytosis / cerebellar granule cell precursor proliferation / positive regulation of myoblast proliferation / positive regulation of meiotic cell cycle / lung lobe morphogenesis / positive regulation of myelination / negative regulation of androgen receptor signaling pathway / cell activation / insulin-like growth factor II binding / positive regulation of developmental growth / glial cell differentiation / positive regulation of calcineurin-NFAT signaling cascade / male sex determination / insulin receptor complex / prostate gland growth / transmembrane receptor protein tyrosine kinase activator activity / insulin-like growth factor I binding / positive regulation of protein-containing complex disassembly / type B pancreatic cell proliferation / insulin receptor activity / mammary gland development / exocrine pancreas development / alphav-beta3 integrin-IGF-1-IGF1R complex / myoblast differentiation / cell surface receptor signaling pathway via STAT / regulation of nitric oxide biosynthetic process / positive regulation of Ras protein signal transduction / regulation of JNK cascade / positive regulation of insulin-like growth factor receptor signaling pathway / dendritic spine maintenance / cargo receptor activity / positive regulation of smooth muscle cell migration / peptidyl-tyrosine autophosphorylation / insulin binding / growth hormone receptor signaling pathway / positive regulation of DNA binding / negative regulation of interleukin-1 beta production / adrenal gland development / neuronal cell body membrane / PTB domain binding / lung alveolus development / cellular response to insulin-like growth factor stimulus / muscle organ development / Signaling by Insulin receptor / IRS activation / positive regulation of cardiac muscle hypertrophy / branching morphogenesis of an epithelial tube / androgen receptor signaling pathway / prostate epithelial cord arborization involved in prostate glandular acinus morphogenesis / negative regulation of release of cytochrome c from mitochondria / type I pneumocyte differentiation / negative regulation of amyloid-beta formation / negative regulation of smooth muscle cell apoptotic process / positive regulation of respiratory burst / positive regulation of activated T cell proliferation / amyloid-beta clearance / inner ear development / Respiratory syncytial virus (RSV) attachment and entry / myoblast proliferation / regulation of embryonic development / positive regulation of receptor internalization / insulin receptor substrate binding / negative regulation of tumor necrosis factor production / protein kinase activator activity / epithelial to mesenchymal transition / blood vessel remodeling / Synthesis, secretion, and deacylation of Ghrelin / epidermis development / activation of protein kinase B activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Xu Y / Lawrence MC | |||||||||
| Funding support |  Australia, 1 items Australia, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2022 Journal: Structure / Year: 2022Title: How insulin-like growth factor I binds to a hybrid insulin receptor type 1 insulin-like growth factor receptor. Authors: Yibin Xu / Mai B Margetts / Hari Venugopal / John G Menting / Nicholas S Kirk / Tristan I Croll / Carlie Delaine / Briony E Forbes / Michael C Lawrence /   Abstract: Monomers of the insulin receptor and type 1 insulin-like growth factor receptor (IGF-1R) can combine stochastically to form heterodimeric hybrid receptors. These hybrid receptors display ligand ...Monomers of the insulin receptor and type 1 insulin-like growth factor receptor (IGF-1R) can combine stochastically to form heterodimeric hybrid receptors. These hybrid receptors display ligand binding and signaling properties that differ from those of the homodimeric receptors. Here, we describe the cryoelectron microscopy structure of such a hybrid receptor in complex with insulin-like growth factor I (IGF-I). The structure (ca. 3.7 Å resolution) displays a single IGF-I ligand, bound in a similar fashion to that seen for IGFs in complex with IGF-1R. The IGF-I ligand engages the first leucine-rich-repeat domain and cysteine-rich region of the IGF-1R monomer (rather than those of the insulin receptor monomer), consistent with the determinants for IGF binding residing in the IGF-1R cysteine-rich region. The structure broadens our understanding of this receptor family and assists in delineating the key structural motifs involved in binding their respective ligands. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24791.map.gz emd_24791.map.gz | 122.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24791-v30.xml emd-24791-v30.xml emd-24791.xml emd-24791.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24791.png emd_24791.png | 85.8 KB | ||
| Filedesc metadata |  emd-24791.cif.gz emd-24791.cif.gz | 6.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24791 http://ftp.pdbj.org/pub/emdb/structures/EMD-24791 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24791 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24791 | HTTPS FTP |
-Validation report
| Summary document |  emd_24791_validation.pdf.gz emd_24791_validation.pdf.gz | 475.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24791_full_validation.pdf.gz emd_24791_full_validation.pdf.gz | 475.1 KB | Display | |
| Data in XML |  emd_24791_validation.xml.gz emd_24791_validation.xml.gz | 6.9 KB | Display | |
| Data in CIF |  emd_24791_validation.cif.gz emd_24791_validation.cif.gz | 8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24791 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24791 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24791 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24791 | HTTPS FTP |
-Related structure data
| Related structure data |  7s0qMC  7s8vC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24791.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24791.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Head region of a complex of IGF-I with the ectodomain of a hybrid insulin receptor / type 1 insulin-like growth factor receptor | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : a complex of IGF-I with the ectodomain of a hybrid insulin recept...
| Entire | Name: a complex of IGF-I with the ectodomain of a hybrid insulin receptor / type 1 insulin-like growth factor receptor |
|---|---|
| Components |
|
-Supramolecule #1: a complex of IGF-I with the ectodomain of a hybrid insulin recept...
| Supramolecule | Name: a complex of IGF-I with the ectodomain of a hybrid insulin receptor / type 1 insulin-like growth factor receptor type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 250 kDa/nm |
-Macromolecule #1: Insulin-like growth factor 1 receptor
| Macromolecule | Name: Insulin-like growth factor 1 receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 108.937242 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EICGPGIDIR NDYQQLKRLE NCTVIEGYLH ILLISKAEDY RSYRFPKLTV ITEYLLLFRV AGLESLGDLF PNLTVIRGWK LFYNYALVI FEMTNLKDIG LYNLRNITRG AIRIEKNADL CYLSTVDWSL ILDAVSNNYI VGNKPPKECG DLCPGTMEEK P MCEKTTIN ...String: EICGPGIDIR NDYQQLKRLE NCTVIEGYLH ILLISKAEDY RSYRFPKLTV ITEYLLLFRV AGLESLGDLF PNLTVIRGWK LFYNYALVI FEMTNLKDIG LYNLRNITRG AIRIEKNADL CYLSTVDWSL ILDAVSNNYI VGNKPPKECG DLCPGTMEEK P MCEKTTIN NEYNYRCWTT NRCQKMCPST CGKRACTENN ECCHPECLGS CSAPDNDTAC VACRHYYYAG VCVPACPPNT YR FEGWRCV DRDFCANILS AESSDSEGFV IHDGECMQEC PSGFIRNGSQ SMYCIPCEGP CPKVCEEEKK TKTIDSVTSA QML QGCTIF KGNLLINIRR GNNIASELEN FMGLIEVVTG YVKIRHSHAL VSLSFLKNLR LILGEEQLEG NYSFYVLDNQ NLQQ LWDWD HRNLTIKAGK MYFAFNPKLC VSEIYRMEEV TGTKGRQSKG DINTRNNGER ASCESDVLHF TSTTTSKNRI IITWH RYRP PDYRDLISFT VYYKEAPFKN VTEYDGQDAC GSNSWNMVDV DLPPNKDVEP GILLHGLKPW TQYAVYVKAV TLTMVE NDH IRGAKSEILY IRTNASVPSI PLDVLSASNS SSQLIVKWNP PSLPNGNLSY YIVRWQRQPQ DGYLYRHNYC SKDKIPI RK YADGTIDIEE VTENPKTEVC GGEKGPCCAC PKTEAEKQAE KEEAEYRKVF ENFLHNSIFV PRPERKRRDV MQVANTTM S SRSRNTTAAD TYNITDPEEL ETEYPFFESR VDNKERTVIS NLRPFTLYRI DIHSCNHEAE KLGCSASNFV FARTMPAEG ADDIPGPVTW EPRPENSIFL KWPEPENPNG LILMYEIKYG SQVEDQRECV SRQEYRKYGG AKLNRLNPGN YTARIQATSL SGNGSWTDP VFFYVQAKTG YENFIHRMKQ LEDKVEELLS KNYHLENEVA RLKKLVGERS SSEQKLISEE DLN UniProtKB: Insulin-like growth factor 1 receptor |
-Macromolecule #2: Insulin receptor
| Macromolecule | Name: Insulin receptor / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 109.809617 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HLYPGEVCPG MDIRNNLTRL HELENCSVIE GHLQILLMFK TRPEDFRDLS FPKLIMITDY LLLFRVYGLE SLKDLFPNLT VIRGSRLFF NYALVIFEMV HLKELGLYNL MNITRGSVRI EKNNELCYLA TIDWSRILDS VEDNYIVLNK DDNEECGDIC P GTAKGKTN ...String: HLYPGEVCPG MDIRNNLTRL HELENCSVIE GHLQILLMFK TRPEDFRDLS FPKLIMITDY LLLFRVYGLE SLKDLFPNLT VIRGSRLFF NYALVIFEMV HLKELGLYNL MNITRGSVRI EKNNELCYLA TIDWSRILDS VEDNYIVLNK DDNEECGDIC P GTAKGKTN CPATVINGQF VERCWTHSHC QKVCPTICKS HGCTAEGLCC HSECLGNCSQ PDDPTKCVAC RNFYLDGRCV ET CPPPYYH FQDWRCVNFS FCQDLHHKCK NSRRQGCHQY VIHNNKCIPE CPSGYTMNSS NLLCTPCLGP CPKVCHLLEG EKT IDSVTS AQELRGCTVI NGSLIINIRG GNNLAAELEA NLGLIEEISG YLKIRRSYAL VSLSFFRKLR LIRGETLEIG NYSF YALDN QNLRQLWDWS KHNLTITQGK LFFHYNPKLC LSEIHKMEEV SGTKGRQERN DIALKTNGDQ ASCENELLKF SYIRT SFDK ILLRWEPYWP PDFRDLLGFM LFYKEAPYQN VTEFDGQDAC GSNSWTVVDI DPPLRSNDPK SQNHPGWLMR GLKPWT QYA IFVKTLVTFS DERRTYGAKS DIIYVQTDAT NPSVPLDPIS VSNSSSQIIL KWKPPSDPNG NITHYLVFWE RQAEDSE LF ELDYCLKGLK LPSRTWSPPF ESEDSQKHNQ SEYEDSAGEC CSCPKTDSQI LKELEESSFR KTFEDYLHNV VFVPRKTS S GTGAEDPRPS RKRRSLGDVG NVTVAVPTVA AFPNTSSTSV PTSPEEHRPF EKVVNKESLV ISGLRHFTGY RIELQACNQ DTPEERCSVA AYVSARTMPE AKADDIVGPV THEIFENNVV HLMWQEPKEP NGLIVLYEVS YRRYGDEELH LCVSRKHFAL ERGCRLRGL SPGNYSVRIR ATSLAGNGSW TEPTYFYVTD YLDVPSNIAR MKQLEDKVEE LLSKNYHLEN EVARLKKLVG E R UniProtKB: Insulin receptor |
-Macromolecule #3: Insulin-like growth factor I
| Macromolecule | Name: Insulin-like growth factor I / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.663752 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPETLCGAEL VDALQFVCGD RGFYFNKPTG YGSSSRRAPQ TGIVDECCFR SCDLRRLEMY CAPLKPAKSA UniProtKB: Insulin-like growth factor 1 |
-Macromolecule #6: beta-D-mannopyranose
| Macromolecule | Name: beta-D-mannopyranose / type: ligand / ID: 6 / Number of copies: 1 / Formula: BMA |
|---|---|
| Molecular weight | Theoretical: 180.156 Da |
| Chemical component information | 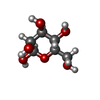 ChemComp-BMA: |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 7 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 / Component - Concentration: 20.0 mM / Component - Formula: C4H11NO3 / Component - Name: TRIS / Details: 20mM Tris pH 8.0 160mM NaCl |
|---|---|
| Grid | Material: COPPER / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
| Details | 0.2mg/ml |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.44 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7s0q: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















