[English] 日本語
 Yorodumi
Yorodumi- EMDB-24784: Cryo-EM map of human GlcNAc-1-phosphotransferase A2B2 subcomplex -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of human GlcNAc-1-phosphotransferase A2B2 subcomplex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GlcNAc-1-phosphotransferase / lysosomal hydrolases / mannose 6-phosphate trafficking pathway / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationUDP-N-acetylglucosamine-lysosomal-enzyme N-acetylglucosaminephosphotransferase / N-glycan processing to lysosome / UDP-N-acetylglucosamine-lysosomal-enzyme N-acetylglucosaminephosphotransferase activity / secretion of lysosomal enzymes / carbohydrate phosphorylation / lysosome organization / Golgi membrane / calcium ion binding / Golgi apparatus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
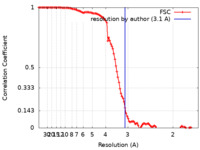
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Li H | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Structure of the human GlcNAc-1-phosphotransferase αβ subunits reveals regulatory mechanism for lysosomal enzyme glycan phosphorylation. Authors: Hua Li / Wang-Sik Lee / Xiang Feng / Lin Bai / Benjamin C Jennings / Lin Liu / Balraj Doray / William M Canfield / Stuart Kornfeld / Huilin Li /   Abstract: Vertebrates use the mannose 6-phosphate (M6P)-recognition system to deliver lysosomal hydrolases to lysosomes. Key to this pathway is N-acetylglucosamine (GlcNAc)-1-phosphotransferase (PTase) that ...Vertebrates use the mannose 6-phosphate (M6P)-recognition system to deliver lysosomal hydrolases to lysosomes. Key to this pathway is N-acetylglucosamine (GlcNAc)-1-phosphotransferase (PTase) that selectively adds GlcNAc-phosphate (P) to mannose residues of hydrolases. Human PTase is an αβγ heterohexamer with a catalytic core and several peripheral domains that recognize and bind substrates. Here we report a cryo-EM structure of the catalytic core of human PTase and the identification of a hockey stick-like motif that controls activation of the enzyme. Movement of this motif out of the catalytic pocket is associated with a rearrangement of part of the peripheral domains that unblocks hydrolase glycan access to the catalytic site, thereby activating PTase. We propose that PTase fluctuates between inactive and active states in solution, and selective substrate binding of a lysosomal hydrolase through its protein-binding determinant to PTase locks the enzyme in the active state to permit glycan phosphorylation. This mechanism would help ensure that only N-linked glycans of lysosomal enzymes are phosphorylated. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24784.map.gz emd_24784.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24784-v30.xml emd-24784-v30.xml emd-24784.xml emd-24784.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24784_fsc.xml emd_24784_fsc.xml | 11.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_24784.png emd_24784.png | 164.2 KB | ||
| Filedesc metadata |  emd-24784.cif.gz emd-24784.cif.gz | 6.6 KB | ||
| Others |  emd_24784_half_map_1.map.gz emd_24784_half_map_1.map.gz emd_24784_half_map_2.map.gz emd_24784_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24784 http://ftp.pdbj.org/pub/emdb/structures/EMD-24784 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24784 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24784 | HTTPS FTP |
-Related structure data
| Related structure data |  7s05MC  7s06C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24784.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24784.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
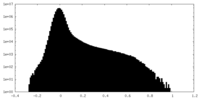
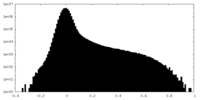
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_24784_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
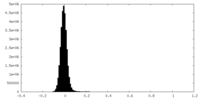
| Density Histograms |
-Half map: #1
| File | emd_24784_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
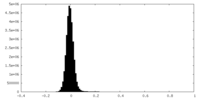
| Density Histograms |
- Sample components
Sample components
-Entire : GlcNAc-1-phosphotransferase
| Entire | Name: GlcNAc-1-phosphotransferase |
|---|---|
| Components |
|
-Supramolecule #1: GlcNAc-1-phosphotransferase
| Supramolecule | Name: GlcNAc-1-phosphotransferase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: N-acetylglucosamine-1-phosphotransferase subunits alpha/beta
| Macromolecule | Name: N-acetylglucosamine-1-phosphotransferase subunits alpha/beta type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: UDP-N-acetylglucosamine-lysosomal-enzyme N-acetylglucosaminephosphotransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 134.7875 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DEDQVDPRLI DGKWSRDQYH VLFDSYRDNI AGKSFQNRLC LPMPIDVVYT WVNGTDLELL KELQQVREQM EEEQKAMREI LGKNTTEPT KKSEKQLECL LTHCIKVPML VLDPALPANI TLKDLPSLYP SFHSASDIFN VAKPKNPSTN VSVVVFDSTK D VEDAHSGL ...String: DEDQVDPRLI DGKWSRDQYH VLFDSYRDNI AGKSFQNRLC LPMPIDVVYT WVNGTDLELL KELQQVREQM EEEQKAMREI LGKNTTEPT KKSEKQLECL LTHCIKVPML VLDPALPANI TLKDLPSLYP SFHSASDIFN VAKPKNPSTN VSVVVFDSTK D VEDAHSGL LKGNSRQTVW RGYLTTDKEV PGLVLMQDLA FLSGFPPTFK ETNQLKTKLP ENLSSKVKLL QLYSEASVAL LK LNNPKDF QELNKQTKKN MTIDGKELTI SPAYLLWDLS AISQSKQDED ISASRFEDNE ELRYSLRSIE RHAPWVRNIF IVT NGQIPS WLNLDNPRVT IVTHQDVFRN LSHLPTFSSP AIESHIHRIE GLSQKFIYLN DDVMFGKDVW PDDFYSHSKG QKVY LTWPV PNCAEGCPGS WIKDGYCDKA CNNSACDWDG GDCSGNSGGS RYIAGGGGTG SIGVGQPWQF GGGINSVSYC NQGCA NSWL ADKFCDQACN VLSCGFDAGD CGQDHFHELY KVILLPNQTH YIIPKGECLP YFSFAEVAKR GVEGAYSDNP IIRHAS IAN KWKTIHLIMH SGMNATTIHF NLTFQNTNDE EFKMQITVEV DTREGPKLNS TAQKGYENLV SPITLLPEAE ILFEDIP KE KRFPKFKRHD VNSTRRAQEE VKIPLVNISL LPKDAQLSLN TLDLQLEHGD ITLKGYNLSK SALLRSFLMN SQHAKIKN Q AIITDETNDS LVAPQEKQVH KSILPNSLGV SERLQRLTFP AVSVKVNGHD QGQNPPLDLE TTARFRVETH TQKTIGGNV TKEKPPSLIV PLESQMTKEK KITGKEKENS RMEENAENHI GVTEVLLGRK LQHYTDSYLG FLPWEKKKYF QDLLDEEESL KTQLAYFTD SKNRARYKRD TFADSLRYVN KILNSKFGFT SRKVPAHMPH MIDRIVMQEL QDMFPEEFDK TSFHKVRHSE D MQFAFSYF YYLMSAVQPL NISQVFDEVD TDQSGVLSDR EIRTLATRIH ELPLSLQDLT GLEHMLINCS KMLPADITQL NN IPPTQES YYDPNLPPVT KSLVTNCKPV TDKIHKAYKD KNKYRFEIMG EEEIAFKMIR TNVSHVVGQL DDIRKNPRKF VCL NDNIDH NHKDAQTVKA VLRDFYESMF PIPSQFELPR EYRNRFLHMH ELQEWRAYRD KLK UniProtKB: N-acetylglucosamine-1-phosphotransferase subunits alpha/beta |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 Component:
| ||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 299 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 193.0 K / Max: 193.0 K |
| Alignment procedure | Coma free - Residual tilt: 0.05 mrad |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 13320 / Average exposure time: 1.5 sec. / Average electron dose: 66.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)