[English] 日本語
 Yorodumi
Yorodumi- EMDB-23746: Cryo-EM structure of zebrafish TRPM5 E337A mutant in the presence... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23746 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of zebrafish TRPM5 E337A mutant in the presence of 5 mM calcium (low calcium occupancy in the transmembrane domain) | ||||||||||||||||||
 Map data Map data | Tetrameric map of zebrafish TRPM5 E337A mutant with low calcium occupancy in the transmembrane domain. The map is only used for docking a tetramer model. The associated subunit map is also provided. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Ion channel / TRP channel / TRANSPORT PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcalcium-activated cation channel activity / calcium channel activity / calcium ion binding / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM | ||||||||||||||||||
 Authors Authors | Ruan Z / Lu W | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition. Authors: Zheng Ruan / Emery Haley / Ian J Orozco / Mark Sabat / Richard Myers / Rebecca Roth / Juan Du / Wei Lü /  Abstract: The Ca-activated TRPM5 channel plays essential roles in taste perception and insulin secretion. However, the mechanism by which Ca regulates TRPM5 activity remains elusive. We report cryo-EM ...The Ca-activated TRPM5 channel plays essential roles in taste perception and insulin secretion. However, the mechanism by which Ca regulates TRPM5 activity remains elusive. We report cryo-EM structures of the zebrafish TRPM5 in an apo closed state, a Ca-bound open state, and an antagonist-bound inhibited state. We define two novel ligand binding sites: a Ca site (Ca) in the intracellular domain and an antagonist site in the transmembrane domain (TMD). The Ca site is unique to TRPM5 and has two roles: modulating the voltage dependence and promoting Ca binding to the Ca site, which is conserved throughout TRPM channels. Conformational changes initialized from both Ca sites cooperatively open the ion-conducting pore. The antagonist NDNA wedges into the space between the S1-S4 domain and pore domain, stabilizing the transmembrane domain in an apo-like closed state. Our results lay the foundation for understanding the voltage-dependent TRPM channels and developing new therapeutic agents. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23746.map.gz emd_23746.map.gz | 135.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23746-v30.xml emd-23746-v30.xml emd-23746.xml emd-23746.xml | 21.9 KB 21.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23746.png emd_23746.png | 148.6 KB | ||
| Filedesc metadata |  emd-23746.cif.gz emd-23746.cif.gz | 7 KB | ||
| Others |  emd_23746_additional_1.map.gz emd_23746_additional_1.map.gz emd_23746_additional_2.map.gz emd_23746_additional_2.map.gz | 11.9 MB 139.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23746 http://ftp.pdbj.org/pub/emdb/structures/EMD-23746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23746 | HTTPS FTP |
-Related structure data
| Related structure data |  7mbtMC  7mbpC  7mbqC  7mbrC  7mbsC  7mbuC  7mbvC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23746.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23746.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Tetrameric map of zebrafish TRPM5 E337A mutant with low calcium occupancy in the transmembrane domain. The map is only used for docking a tetramer model. The associated subunit map is also provided. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Sharpened single subunit map for zebrafish TRPM5 E337A...
| File | emd_23746_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened single subunit map for zebrafish TRPM5 E337A mutant with low calcium occupancy at transmembrane domain. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Non-sharpened single subunit map for zebrafish TRPM5 E337A...
| File | emd_23746_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Non-sharpened single subunit map for zebrafish TRPM5 E337A mutant with low calcium occupancy at transmembrane domain. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TRPM5 channel E337A mutant
| Entire | Name: TRPM5 channel E337A mutant |
|---|---|
| Components |
|
-Supramolecule #1: TRPM5 channel E337A mutant
| Supramolecule | Name: TRPM5 channel E337A mutant / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Transient receptor potential melastatin 5
| Macromolecule | Name: Transient receptor potential melastatin 5 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 132.740953 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVEKSSERFD KQMAGRLGDI DFTGVSRTRG KFVRVTSSTD PAEIYQILTK QWGLAPPHLV VALMGGDEVA QLKPWLRDTL RKGLVKAAQ STGAWILTSG LRFGITKNLG QAVRDHSLAS TSPKVRVVAI GIAPWNMIQN RDLLLSAKPD HPATYPTEDL P YGAVYSLD ...String: MVEKSSERFD KQMAGRLGDI DFTGVSRTRG KFVRVTSSTD PAEIYQILTK QWGLAPPHLV VALMGGDEVA QLKPWLRDTL RKGLVKAAQ STGAWILTSG LRFGITKNLG QAVRDHSLAS TSPKVRVVAI GIAPWNMIQN RDLLLSAKPD HPATYPTEDL P YGAVYSLD CNHSHFILVD EDPKRPGATG EMRVKMLKHI SLQRTGYGGT GSIEIPVLCL LVHGEPRILQ KMYKNIQNSI PW LILAGSG GVADILVTLM DRGCWDADIV QELLINTFPD GLHSTEITSW TKLIQRILDH GHLLTVHDPE QDSELDTVIL KAL VKACKS QSQEAQDFLD ALKLAVAWNR VDIAKSEIFS GDVQWSAQDL EEVMMEALVN DKPDFVRLFV DNGVNIKQFL TYGR LQELY CSVSEKNLLH TLLLKKNQER QAQLARKRMS GNPNNELGDR KFRFTFHEVS KVLKDFLDDT CKGFYQKLPA ERMGK GRLF HSQKNLPDMD RRCEHPWRDL FLWAILQNRQ EMANYFWAMG PEAVAAALVG CKIMKEMAHL ATEAESARSM KNAKYE QFA MDLFSECYSN SEDRAYSLLV RKTCCWSKAT VLNIATLAEA KCFFAHDGVQ ALLTKVWWGA MRTDTSISRL VLTFFIP PL VWTSLIKFNP EEQVSKDEGE PFAELDSLET EQALLLTDGD PVAGEGSAET AARSCSATFI RVVLRRWNRF WSAPVTVF M GNVIMYFAFL ILFSYVLLLD FRPPPPYGPS AAEIILYFWV FTLVLEEIRQ SFFTDEDMSI LKKMKLYVED NWNKCDMVA ISLFVVGLSC RMAMSTYEAG RTVLALDFMV FTLRLIHIFA IHKQLGPKII IVERMIKDVF FFLFFLSVWL IAYGVTTQAL LHPNDPRID WVFRRALYRP YLHIFGQIPL EEIDAAKMPD DNCTTDVQEI ILGTLPPCPN IYANWLVILL LVIYLLVTNV L LLNLLIAM FSYTFQVVQE NADIFWKFQR YNLIVEYHSR PALAPPFIII SHITQALLSF IKKTENTQDL LERELPSGLD QK LMTWETV QKENYLAKLE HEHRESSGER LRYTSSKVQT LLRMVGGFKD QEKRMATVET EVRYCGEVLS WIAECFHKST LKC DRDAPK APRSIAGSSR DQQPQGAKRQ QPGGHPAYGT DKKLPFIDH UniProtKB: Transient receptor potential cation channel subfamily M member 5 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: (2R)-2-(hydroxymethyl)-4-{[(25R)-10alpha,14beta,17beta-spirost-5-...
| Macromolecule | Name: (2R)-2-(hydroxymethyl)-4-{[(25R)-10alpha,14beta,17beta-spirost-5-en-3beta-yl]oxy}butyl 4-O-alpha-D-glucopyranosyl-beta-D-glucopyranoside type: ligand / ID: 3 / Number of copies: 4 / Formula: YUY |
|---|---|
| Molecular weight | Theoretical: 841.033 Da |
| Chemical component information |  ChemComp-YUY: |
-Macromolecule #4: (25R)-14beta,17beta-spirost-5-en-3beta-ol
| Macromolecule | Name: (25R)-14beta,17beta-spirost-5-en-3beta-ol / type: ligand / ID: 4 / Number of copies: 4 / Formula: YUV |
|---|---|
| Molecular weight | Theoretical: 414.621 Da |
| Chemical component information | 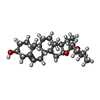 ChemComp-YUV: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 47.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7mbt: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)