+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21708 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cu(I)-bound Copper Storage Protein BsCsp3 | |||||||||
 Map data Map data | BsCSP3 Density Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | small protein (47 kDa) / Z-contrast enhancement in cryo-EM / copper storage protein / metal binding protein | |||||||||
| Function / homology | Protein of unknown function DUF326 / Copper storage protein / Uncharacterized cysteine-rich protein YhjQ-like / Uncharacterized cysteine-rich protein YhjQ Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
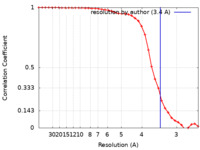
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Chen JZ / Oken A | |||||||||
| Funding support |  United States, United States,  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cu(I)-bound Copper Storage Protein BsCsp3 Authors: Chen JZ / Dennison C | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21708.map.gz emd_21708.map.gz | 1.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21708-v30.xml emd-21708-v30.xml emd-21708.xml emd-21708.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21708_fsc.xml emd_21708_fsc.xml | 4.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_21708.png emd_21708.png | 194.5 KB | ||
| Masks |  emd_21708_msk_1.map emd_21708_msk_1.map | 3.4 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21708.cif.gz emd-21708.cif.gz | 6.2 KB | ||
| Others |  emd_21708_half_map_1.map.gz emd_21708_half_map_1.map.gz emd_21708_half_map_2.map.gz emd_21708_half_map_2.map.gz | 1.7 MB 1.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21708 http://ftp.pdbj.org/pub/emdb/structures/EMD-21708 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21708 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21708 | HTTPS FTP |
-Related structure data
| Related structure data |  6wktMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21708.map.gz / Format: CCP4 / Size: 3.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21708.map.gz / Format: CCP4 / Size: 3.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BsCSP3 Density Map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
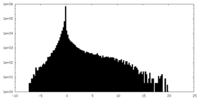
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.296 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
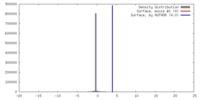
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21708_msk_1.map emd_21708_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
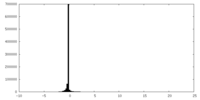
| Projections & Slices |
| ||||||||||||
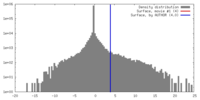
| Density Histograms |
-Half map: BsCSP3 half map - even
| File | emd_21708_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BsCSP3 half map - even | ||||||||||||
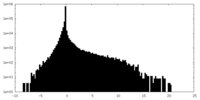
| Projections & Slices |
| ||||||||||||
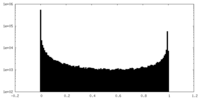
| Density Histograms |
-Half map: BsCSP3 half map - odd
| File | emd_21708_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BsCSP3 half map - odd | ||||||||||||
| Projections & Slices |
| ||||||||||||
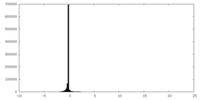
| Density Histograms |
- Sample components
Sample components
-Entire : BsCsp3, Cu(I) ions
| Entire | Name: BsCsp3, Cu(I) ions |
|---|---|
| Components |
|
-Supramolecule #1: BsCsp3, Cu(I) ions
| Supramolecule | Name: BsCsp3, Cu(I) ions / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47 KDa |
-Macromolecule #1: Csp3
| Macromolecule | Name: Csp3 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.853758 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEQYSEACIE ACIDCMKACN HCFTKCLEES VQHHLSGCIR LDRECADICA LAVKAMQTDS PFMKEICALC ADICEACGTE CGKHDHDHC QACAKACFTC AEQCRSMAA UniProtKB: Uncharacterized cysteine-rich protein YhjQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.11 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 15 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.038 kPa / Details: 15 mA | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV Details: 5 micro-liters of sample loaded, waited 1 second,front + back blotted for 4.5 seconds at force 1 before plunging. | |||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 90.0 K / Max: 95.0 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 2212 / Average exposure time: 0.985 sec. / Average electron dose: 40.0 e/Å2 Details: Images were collected at 40 frames per movie. Four movies were collected per stage shift. Frames were collected in super resolution mode at a calibrated pixel size of 0.324 A/pixel. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 2.1 µm / Calibrated defocus min: 0.8 µm / Calibrated magnification: 136000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.15 µm / Nominal defocus min: 0.85 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 4-108 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 46.04 / Target criteria: Correlation coefficient |
| Output model |  PDB-6wkt: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)