[English] 日本語
 Yorodumi
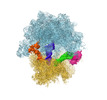
Yorodumi- EMDB-21621: Cryo-EM of elongating ribosome with EF-Tu*GTP elucidates tRNA pro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21621 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM of elongating ribosome with EF-Tu*GTP elucidates tRNA proofreading (Cognate Structure II-A) | ||||||||||||
 Map data Map data | Map II-A blocfilt filtered and with B-factor -50 applied | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Ribosome / EF-Tu / tRNA / Ribosome-RNA complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationguanyl-nucleotide exchange factor complex / protein-synthesizing GTPase / guanosine tetraphosphate binding / translation elongation factor activity / DnaA-L2 complex / negative regulation of DNA-templated DNA replication initiation / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity ...guanyl-nucleotide exchange factor complex / protein-synthesizing GTPase / guanosine tetraphosphate binding / translation elongation factor activity / DnaA-L2 complex / negative regulation of DNA-templated DNA replication initiation / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / GTPase activity / GTP binding / magnesium ion binding / RNA binding / zinc ion binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
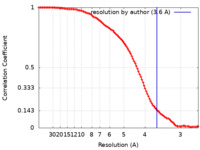
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Loveland AB / Demo G | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Cryo-EM of elongating ribosome with EF-Tu•GTP elucidates tRNA proofreading. Authors: Anna B Loveland / Gabriel Demo / Andrei A Korostelev /   Abstract: Ribosomes accurately decode mRNA by proofreading each aminoacyl-tRNA that is delivered by the elongation factor EF-Tu. To understand the molecular mechanism of this proofreading step it is necessary ...Ribosomes accurately decode mRNA by proofreading each aminoacyl-tRNA that is delivered by the elongation factor EF-Tu. To understand the molecular mechanism of this proofreading step it is necessary to visualize GTP-catalysed elongation, which has remained a challenge. Here we use time-resolved cryogenic electron microscopy to reveal 33 ribosomal states after the delivery of aminoacyl-tRNA by EF-Tu•GTP. Instead of locking cognate tRNA upon initial recognition, the ribosomal decoding centre dynamically monitors codon-anticodon interactions before and after GTP hydrolysis. GTP hydrolysis enables the GTPase domain of EF-Tu to extend away, releasing EF-Tu from tRNA. The 30S subunit then locks cognate tRNA in the decoding centre and rotates, enabling the tRNA to bypass 50S protrusions during accommodation into the peptidyl transferase centre. By contrast, the decoding centre fails to lock near-cognate tRNA, enabling the dissociation of near-cognate tRNA both during initial selection (before GTP hydrolysis) and proofreading (after GTP hydrolysis). These findings reveal structural similarity between ribosomes in initial selection states and in proofreading states, which together govern the efficient rejection of incorrect tRNA. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21621.map.gz emd_21621.map.gz | 85.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21621-v30.xml emd-21621-v30.xml emd-21621.xml emd-21621.xml | 78.5 KB 78.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21621_fsc.xml emd_21621_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_21621.png emd_21621.png | 137.2 KB | ||
| Filedesc metadata |  emd-21621.cif.gz emd-21621.cif.gz | 14.4 KB | ||
| Others |  emd_21621_additional.map.gz emd_21621_additional.map.gz emd_21621_half_map_1.map.gz emd_21621_half_map_1.map.gz emd_21621_half_map_2.map.gz emd_21621_half_map_2.map.gz | 84.5 MB 34 MB 34 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21621 http://ftp.pdbj.org/pub/emdb/structures/EMD-21621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21621 | HTTPS FTP |
-Related structure data
| Related structure data |  6wd2MC  6wd0C  6wd1C  6wd3C  6wd4C  6wd5C  6wd6C  6wd7C  6wd8C  6wd9C  6wdaC  6wdbC  6wdcC  6wddC  6wdeC  6wdfC  6wdgC  6wdhC  6wdiC  6wdjC  6wdkC  6wdlC  6wdmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21621.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21621.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map II-A blocfilt filtered and with B-factor -50 applied | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.333 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Map II-A
| File | emd_21621_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map II-A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 II-A
| File | emd_21621_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 II-A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 II-A
| File | emd_21621_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 II-A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Map II-A
+Supramolecule #1: Map II-A
+Macromolecule #1: 50S ribosomal protein L2
+Macromolecule #2: 50S ribosomal protein L3
+Macromolecule #3: 50S ribosomal protein L4
+Macromolecule #4: 50S ribosomal protein L5
+Macromolecule #5: 50S ribosomal protein L6
+Macromolecule #6: 50S ribosomal protein L9
+Macromolecule #7: 50S ribosomal protein L10
+Macromolecule #8: 50S ribosomal protein L11
+Macromolecule #9: 50S ribosomal protein L13
+Macromolecule #10: 50S ribosomal protein L14
+Macromolecule #11: 50S ribosomal protein L15
+Macromolecule #12: 50S ribosomal protein L16
+Macromolecule #13: 50S ribosomal protein L17
+Macromolecule #14: 50S ribosomal protein L18
+Macromolecule #15: 50S ribosomal protein L19
+Macromolecule #16: 50S ribosomal protein L20
+Macromolecule #17: 50S ribosomal protein L21
+Macromolecule #18: 50S ribosomal protein L22
+Macromolecule #19: 50S ribosomal protein L23
+Macromolecule #20: 50S ribosomal protein L24
+Macromolecule #21: 50S ribosomal protein L25
+Macromolecule #22: 50S ribosomal protein L27
+Macromolecule #23: 50S ribosomal protein L28
+Macromolecule #24: 50S ribosomal protein L29
+Macromolecule #25: 50S ribosomal protein L30
+Macromolecule #26: 50S ribosomal protein L32
+Macromolecule #27: 50S ribosomal protein L33
+Macromolecule #28: 50S ribosomal protein L34
+Macromolecule #29: 50S ribosomal protein L35
+Macromolecule #30: 50S ribosomal protein L36
+Macromolecule #31: 30S ribosomal protein S2
+Macromolecule #32: 30S ribosomal protein S3
+Macromolecule #33: 30S ribosomal protein S4
+Macromolecule #34: 30S ribosomal protein S5
+Macromolecule #35: 30S ribosomal protein S6
+Macromolecule #36: 30S ribosomal protein S7
+Macromolecule #37: 30S ribosomal protein S8
+Macromolecule #38: 30S ribosomal protein S9
+Macromolecule #39: 30S ribosomal protein S10
+Macromolecule #40: 30S ribosomal protein S11
+Macromolecule #41: 30S ribosomal protein S12
+Macromolecule #42: 30S ribosomal protein S13
+Macromolecule #43: 30S ribosomal protein S14
+Macromolecule #44: 30S ribosomal protein S15
+Macromolecule #45: 30S ribosomal protein S16
+Macromolecule #46: 30S ribosomal protein S17
+Macromolecule #47: 30S ribosomal protein S18
+Macromolecule #48: 30S ribosomal protein S19
+Macromolecule #49: 30S ribosomal protein S20
+Macromolecule #50: 30S ribosomal protein S21
+Macromolecule #51: 50S ribosomal protein L1
+Macromolecule #58: Elongation factor Tu
+Macromolecule #52: 16S ribosomal RNA
+Macromolecule #53: 23S ribosomal RNA
+Macromolecule #54: 5S ribosomal RNA
+Macromolecule #55: tRNAfMet
+Macromolecule #56: mRNA
+Macromolecule #57: tRNAPhe
+Macromolecule #59: N-FORMYLMETHIONINE
+Macromolecule #60: PHENYLALANINE
+Macromolecule #61: GUANOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-35 / Number grids imaged: 1 / Number real images: 3218 / Average exposure time: 1.0 sec. / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-6wd2: |
 Movie
Movie Controller
Controller



































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)